Aqueous phase preparation method for water-soluble chiral ZnCdSe quantum dot
A water-phase preparation, water-soluble technology, applied in chemical instruments and methods, nanotechnology, nano-optics and other directions, can solve the problems of cumbersome preparation steps, large amount of cadmium, scarcity, etc., and achieves cheap and easy to obtain raw materials, short reaction time, The effect of high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 1. Prepare KHSe solution: mix 20mg selenium powder and 40mgKBH 4 Mixed, inject 3mL deionized water, stir and react at 25°C for 45min to obtain KHSe solution.
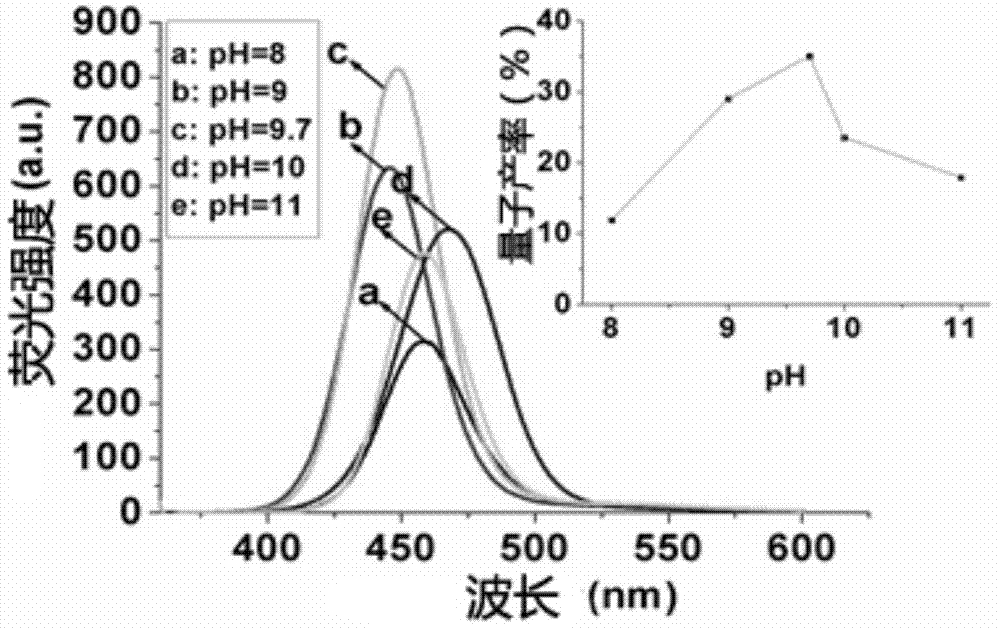
[0054] 2. Prepare the precursor solution of zinc in the two-necked flask, with 24.5mgZnCl 2 and 88.0mg N-acetyl-L-cysteine were dissolved in 50mL deionized water, and the pH value of the solution was adjusted to 8~11 with 1mol / L NaOH to prepare a zinc precursor solution, wherein [Zn 2+ ]=3.6mmol / L; The molar ratio of Zn and N-acetyl-L-cysteine is 1:3.
[0055] 3. Pass argon for 20 minutes to remove the oxygen in the zinc precursor solution, quickly inject 213 μL of the KHSe solution prepared in step 1, and 1 mL of 3.6 mmol / L CdCl 2 solution. After mixing, transfer to a hydrothermal reaction kettle, heat to 200° C. in an oven, and react for 60 minutes to obtain a solution of ZnCdSe quantum dots coated with water-soluble chiral N-acetyl-L-cysteine.
[0056] 4. Add 2 times the volume of ethanol to the obtain...
Embodiment 2
[0058] 1. Prepare KHSe solution: mix 10mg selenium powder and 25mgKBH 4 Mix, inject 3mL of deionized water, and stir the reaction at 20°C for 46min to obtain a KHSe solution.
[0059] 2. Prepare the precursor solution of zinc in the two-necked flask, with 24.5mgZnCl 2 and 88.0mg N-acetyl-L-cysteine were dissolved in 50mL deionized water, and the pH value of the solution was adjusted to 9.7 with 1mol / L NaOH to prepare a zinc precursor solution, wherein [Zn 2+ ]=3.6mmol / L; The molar ratio of Zn and N-acetyl-L-cysteine is 1:3.
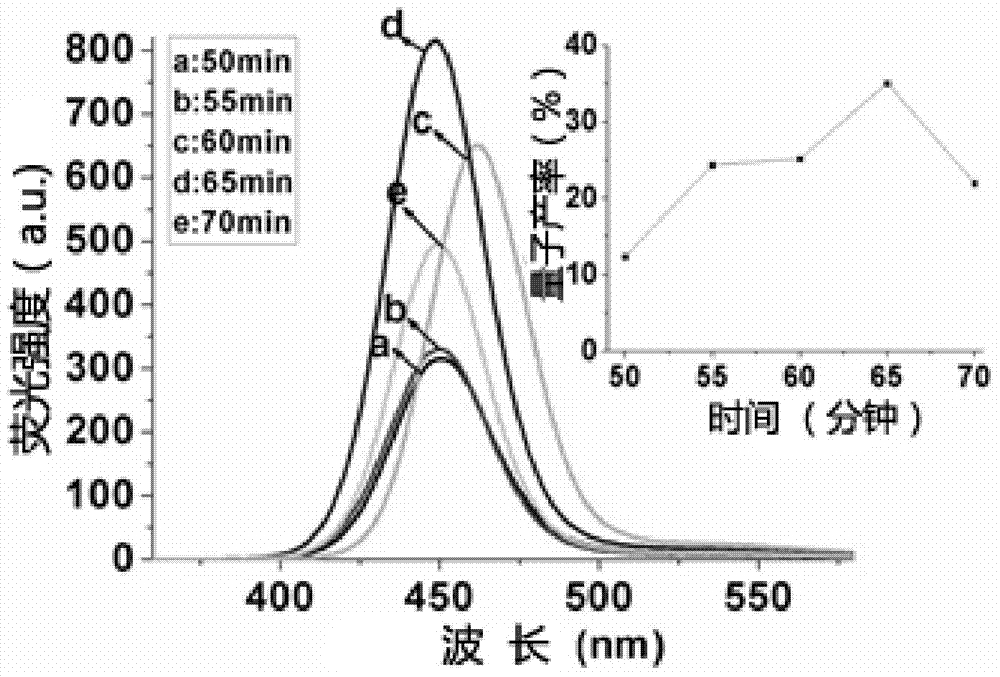
[0060] 3. Nitrogen was passed for 35 minutes to remove the oxygen in the zinc precursor solution, and 426 μL of the KHSe solution prepared in step 1 and 1 mL of 3.6 mmol / L CdCl were quickly injected 2 solution. After mixing, transfer to a hydrothermal reaction kettle, heat to 200° C. in an oven, and react for 50 to 70 minutes to obtain a water-soluble chiral N-acetyl-L-cysteine-wrapped ZnCdSe quantum dot solution.
[0061] 4. Add 2 times the volum...
Embodiment 3
[0063] 1. Prepare NaHSe solution: mix 10mg selenium powder and 11mgNaBH 4 After mixing, inject 3 mL of deionized water, and stir the reaction at 15°C for 100 min to obtain a NaHSe solution.
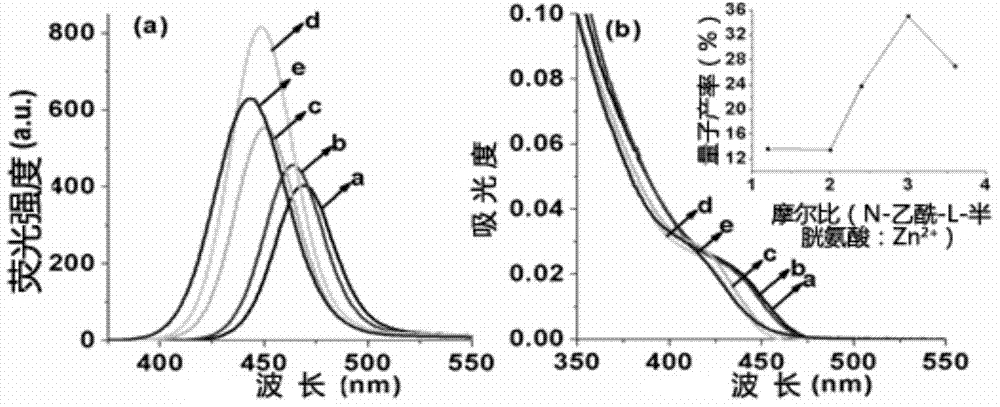
[0064] 2. Prepare the precursor solution of zinc in the two-necked flask, with 24.5mgZnCl 2 35.2 mg, 58.7 mg, 70.4 mg, 88.0 mg and 105.6 mg of N-acetyl-L-cysteine were mixed and dissolved in 50 mL of deionized water, and the pH value of the solution was adjusted to 9.7 with 1 mol / L NaOH to prepare Get the precursor solution of zinc, wherein [Zn 2+]=3.6mmol / L; the molar ratios of Zn and N-acetyl-L-cysteine were 1:1.2, 1:2.0, 1:2.4, 1:3.0, 1:3.6, respectively.
[0065] 3. Nitrogen gas was passed for 40 minutes to remove the oxygen in the zinc precursor solution, and 426 μL of the NaHSe solution prepared in step 1 and 1 mL of 3.6 mmol / L CdCl were quickly injected 2 solution. After mixing, transfer to a hydrothermal reaction kettle, heat to 200° C. in an oven, and react for 60 minutes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com