Pteris semipinnata general flavone sustained release preparation and preparation method thereof
A technology of sustained-release preparations and total flavonoids, which is applied in pharmaceutical formulations, medical raw materials derived from ferns/filamentous plants, drug delivery, etc., can solve life-threatening problems, achieve balanced and lasting curative effect, and overcome frequent medication , the effect of enhancing compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
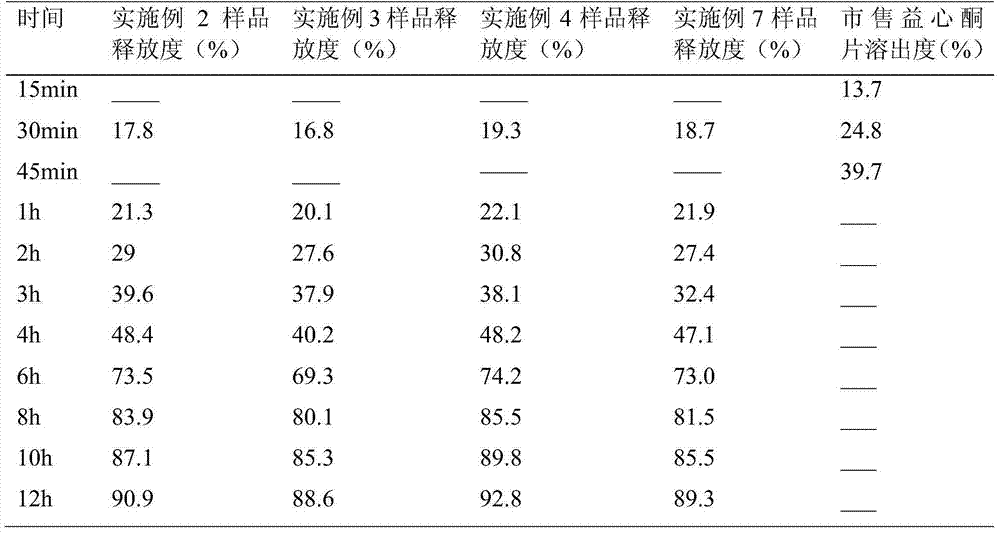
[0023] Respectively, the total flavonoids of Banner Banner, carboxypropyl cellulose K1001v, microcrystalline cellulose and magnesium stearate are fully dried and passed through a 100-mesh sieve for subsequent use; take 8 g of Banner Banner total flavonoids, add 13.5 g of carboxypropyl methylcellulose K1001v and 8.2g microcrystalline cellulose, mix well, add 5.94ml of 90% ethanol to prepare soft material, granulate with 20 mesh sieve, dry at 60°C, then granulate with 24 mesh sieve, add 0.3g magnesium stearate and mix evenly, 100 sustained-release tablets were prepared by a tablet machine.
Embodiment 2
[0025] Respectively fully dry the total flavonoids of Banner Banner, carboxypropyl cellulose K4M, microcrystalline cellulose, sodium carboxymethyl cellulose, and magnesium stearate, pass through a 100-mesh sieve, and set aside; take 8g of total flavonoids of Banner Banner, add 12.0g Carmellose K4M, 8.2g of microcrystalline cellulose and 1.5g of sodium carboxymethylcellulose were mixed uniformly, and 5.94ml of 90% ethanol was added to prepare a soft material, granulated with a 20-mesh sieve, dried at 60°C, and then dried at 24 Mesh sieve for granulation, add 0.3g of magnesium stearate and mix evenly, prepare 100 sustained-release tablets through a tablet machine.
Embodiment 3
[0027] Fully dry the total flavonoids, carboxypropyl cellulose K4M, microcrystalline cellulose, and magnesium stearate respectively and pass through a 100-mesh sieve for later use; take 7.6 g of the total flavonoids of the half-pinner, add 12.0 g of carboxypropyl methylcellulose K4M 10.1g microcrystalline cellulose, mix well, add 5.94ml of 90% ethanol to prepare soft material, granulate with 20 mesh sieve, dry at 60°C, then granulate with 24 mesh sieve, add 0.3g magnesium stearate and mix evenly , prepare 100 sustained-release tablets through a tablet machine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com