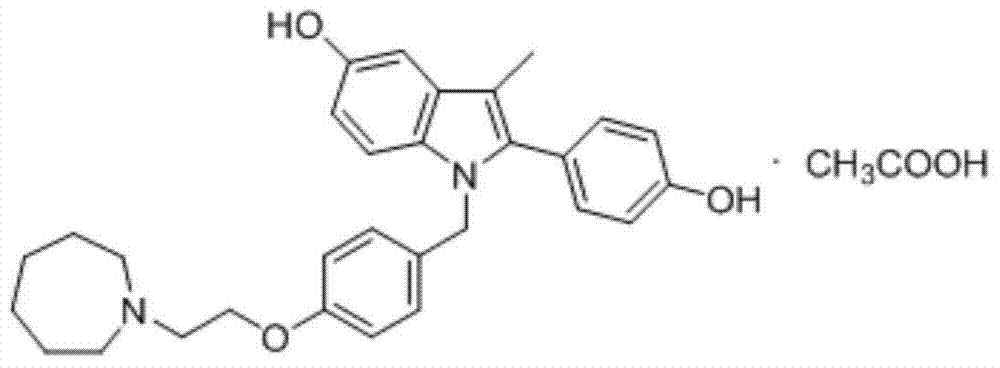
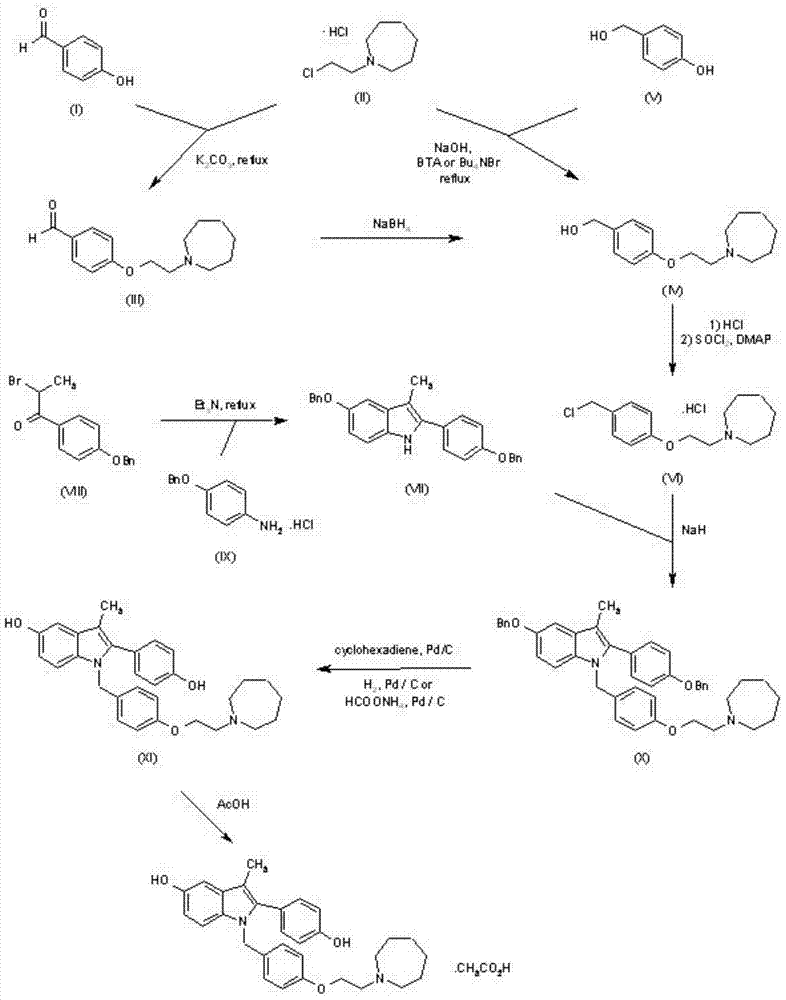
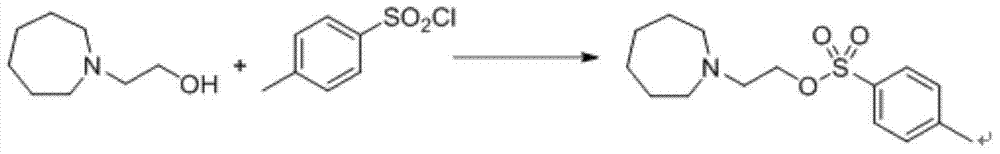
Preparation method of (4-(2-azacycloheptane-1-yl)ethoxy)phenyl)methanol
A technology of azepane and ethoxy, which is applied in the field of drug synthesis, can solve the problems of good product quality, high toxicity of raw materials, and high yield, and achieve the effect of good product quality, high toxicity of raw materials, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of 2-(azepan-1-yl)ethyl-4-methylbenzenesulfonate
[0022] Weigh 2.15 g of 2-(azepane)-1-ethanol (15.0 mmol) and mix with 20 ml of anhydrous pyridine, stir to cool to 0°C, and add 2.86 g of p-toluenesulfonyl chloride (15.0 mmol). After the addition, react at room temperature for 3 hours, add water to quench the reaction, continue to stir for 30 minutes, add dichloromethane, add 1M hydrochloric acid dropwise under stirring to adjust the pH=6-7, stir for 30 minutes and then let it stand for stratification. The methane layer was washed with water and saturated brine successively, then dried (anhydrous sodium sulfate), filtered, and dichloromethane was distilled off under reduced pressure to obtain a purple oil, which was directly used in the next reaction without further purification.
Embodiment 2
[0023] Embodiment two: the preparation of 4-(2-(azepan-1-yl)ethoxy)benzaldehyde
[0024] A certain amount of 2-(azepan-1-yl)ethyl-4-methylbenzenesulfonate and an equimolar amount of 4-formylphenol sodium were mixed in DMF, stirred and heated to 80°C, and monitored The response is complete. After the reaction is completed, cool to room temperature, pour the reaction solution into water, add dichloromethane to extract, combine the dichloromethane phases, wash with water and saturated brine successively, dry (anhydrous magnesium sulfate), filter, and distill off the dichloromethane, leaving The product was purified by column chromatography (n-hexane / ethyl acetate=98:2) to obtain 4-(2-(azepan-1-yl)ethoxy)benzaldehyde as a colorless oil with a yield of 80% . IR (nujol): 2926, 2853, 1695, 1603, 1507, 1312, 1259, 1160, 1021 and 834cm -1 . 1 H NMR (300MHz, CDCl 3 ): δ1.65-1.70(8H,m,4×CH2),2.87(4H,brs,2×NCH 2 ),3.06(2H,t,NCH2),4.22(2H,t,OCH 2 ), 7.05 (2H, d, 2×Ar-H), 7.86 (2H, d...
Embodiment 3
[0025] Example 3: Preparation of (4-(2-(azepan-1-yl)ethoxy)phenyl)methanol
[0026] Mix 4.5mmol of the reaction product of the previous step with 25ml of methanol, add 3.1mmol of sodium borohydride in batches at room temperature, and react for 3-5 hours at room temperature after addition, monitor the reaction by TLC (developer: methanol / dichloromethane=1: 9). After the reaction was completed, methanol was distilled off under reduced pressure, and 15ml of water was added to the residue, extracted 3 times with ethyl acetate (50ml x 3 times), the combined ethyl acetate phase was washed with water 3 times (50ml x 3 times), dried (anhydrous sodium sulfate), filtered, and distilled off ethyl acetate under reduced pressure to obtain an oily product with a yield of 80-90%. IR (nujol): 3360, 2934, 2867, 1610, 1513, 1456, 1325, 1300, 1247, 1176, 1055, 1010, 823cm -1 . 1 HNMR (CDCl 3 / TMS):7.28(d,2H),6.86(d,2H),4.61(s,2H),4.06(t,2H),2.94(t,2H),2.76(m,4H),1.7-1.5( m, 8H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com