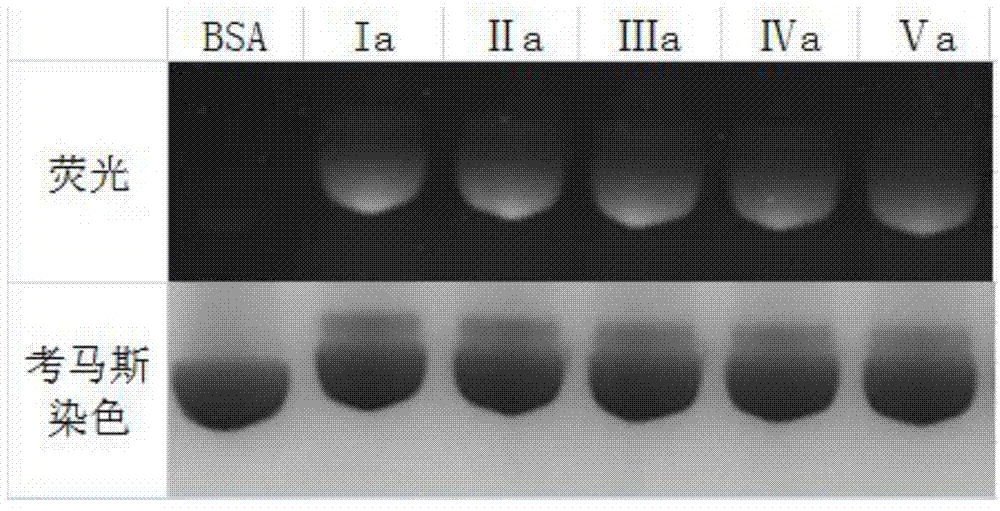
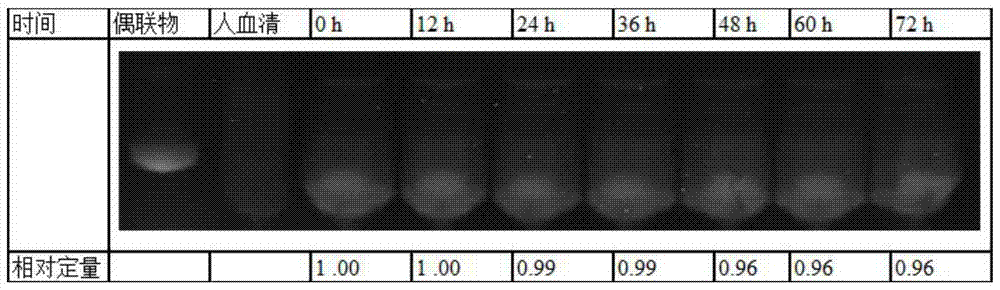
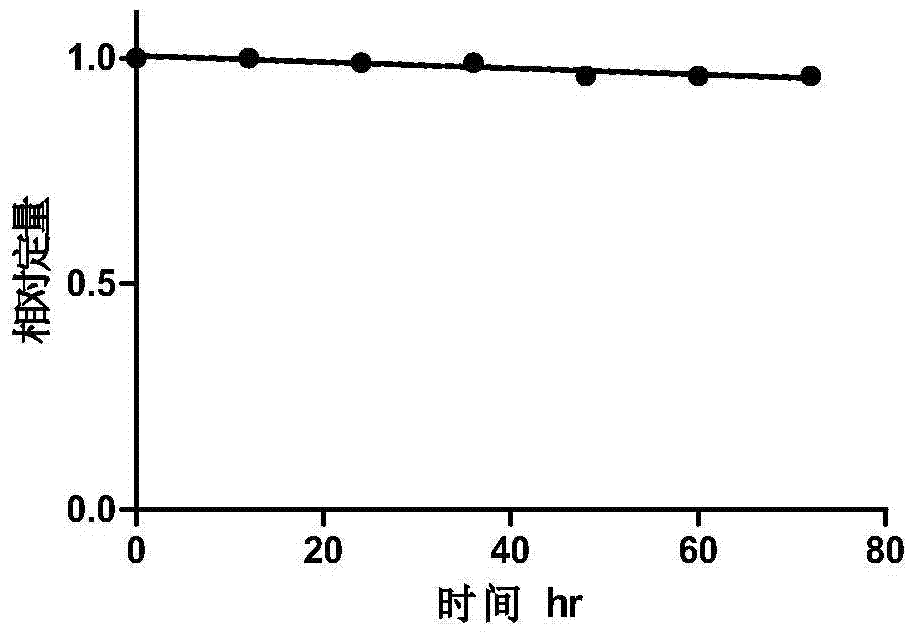
Fluorescent labeled molecule with alkenyl sulfonyl groups and method for labeling protein by virtue of fluorescent labeled molecule
A technology of alkenylsulfonyl group and fluorescent labeling, which is applied in peptide preparation methods, chemical instruments and methods, luminescent materials, etc., can solve the problems of poor stability of linking molecules or protein conjugates, and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: the synthesis of compound (Ia)
[0056]
[0057] The synthetic route is as follows:
[0058]
[0059] Concrete synthetic steps are as follows:
[0060] (1) Compound 2a (i.e. coumarin 3-carboxylate) (1.9g, 10mmol) was dissolved in 15mL SOCl 2 , the reaction solution was refluxed for 1 hour, and the remaining SOCl 2 , the obtained product compound 4a was directly subjected to the next reaction without purification.
[0061] (2) Add compound 4a prepared above to a solution of compound 3a (3g, 12mmol) and triethylamine (2mL, 15mmol) in dichloromethane under ice bath, and the reaction solution was stirred at room temperature for 10 minutes. Add NaHCO 3 The reaction was quenched with saturated solution and extracted with dichloromethane, and the organic phase was once washed with NaHCO 3 Saturated solution, 1M HCl, and water were washed, dried over anhydrous sodium sulfate, spin-dried, and passed through a column (petroleum ether / ethyl acetate=2:3) to...
Embodiment 2
[0064] Embodiment 2: the synthesis of compound (IIa)
[0065]
[0066] 2-Chloroethanesulfonyl chloride (209 μL, 2 mmol) was added dropwise to a solution of 7-aminocoumarin (134 mg, 0.83 mmol) and triethylamine (0.3 mL, 2 mmol) in 5 mL of dichloromethane, and reacted at room temperature for 5 minutes. Add water to quench the reaction, then extract with dichloromethane, anhydrous Na 2 SO 4 After drying, it was passed through the column to obtain the product Compound (IIa) (164 mg, 79% purification yield) as a colorless liquid. m / z[M+H] + =252.
Embodiment 3
[0067] Embodiment 3: the synthesis of compound (IIIa)
[0068]
[0069] The synthetic route is as follows:
[0070]
[0071] Concrete synthetic steps are as follows:
[0072] (1) Add DCC (412mg, 2mmol) to the ethyl acetate solution of 7-aminocoumarin (compound 8a) (322mg, 2mmol) and compound 9a (494mg, 2mmol), stir at room temperature for one hour, filter, and pass The column gave the product compound 10a (585 mg, 75% purified yield) m / z [M+H] + =363.
[0073] (2) Compound 10a (585mg, 1.79mmol) was dissolved in TFA, the reaction was stirred for 30 minutes, the solvent was spun out, and washed with ether to obtain the product Compound 11a (508mg, 98%) m / z[M+H] + =263.
[0074] (3) Add 2-chloroethanesulfonyl chloride (183 μL, 1.75 mmol) dropwise to a solution of compound 11a (508 mg, 1.75 mmol) and triethylamine (0.25 mL, 1.75 mmol) in 5 mL of dichloromethane, and react at room temperature for 5 minutes . Add water to quench the reaction, then extract with dichlorome...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap