Method for simultaneous preparation of 7-hydroxy-4 '-methoxy flavan and pterostilbene
A technology of methoxyflavan and pterostilbene, which is applied in the field of simultaneous preparation of 7-hydroxy-4'-methoxyflavan and pterostilbene, achieving the effect of a good material basis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
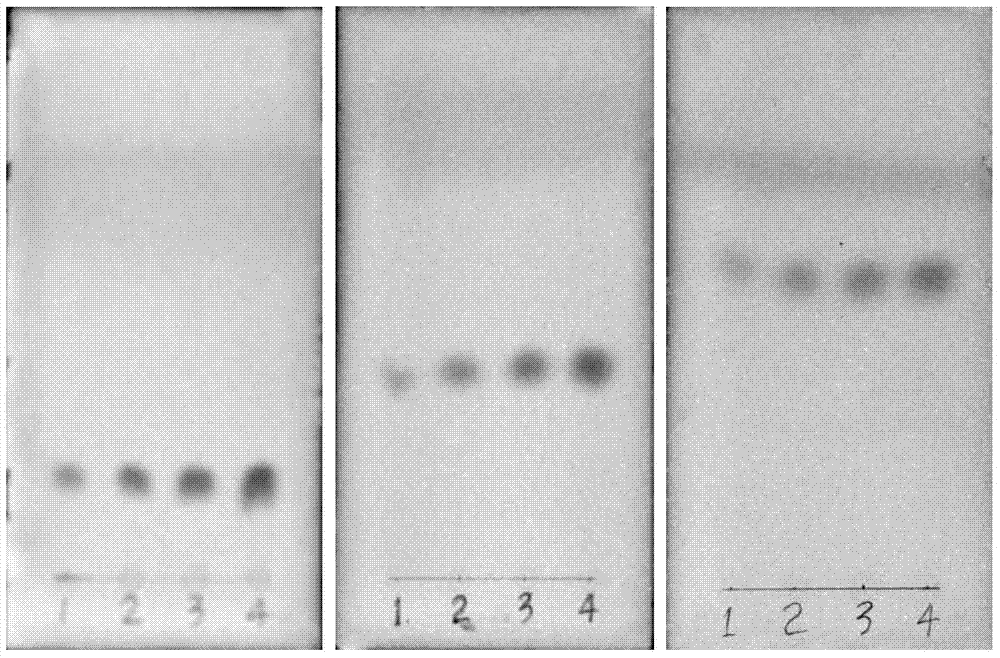
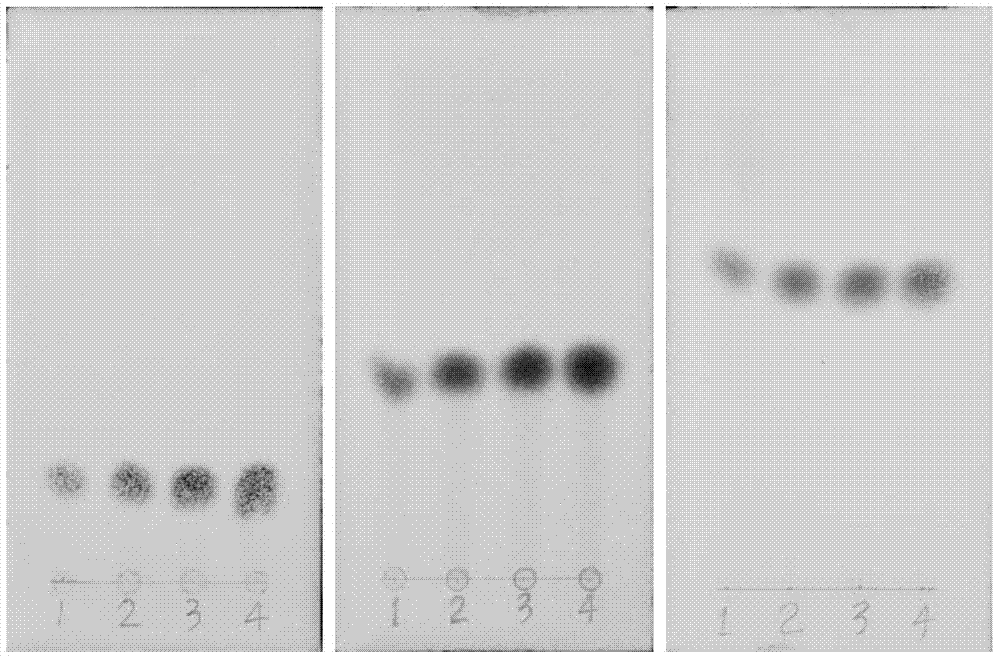
[0050] Get 0.5kg of the resinous wood of Dracaena phoenix leaf, grind it, add 6 times the amount of petroleum ether to extract for 2 days, filter, and use 8 times the amount of chloroform to reflux extract the filter residue for 3 times, recycle the chloroform to obtain the chloroform extract, use 60 Dissolve in % methanol, pass through macroporous resin column chromatography, elute with 60% methanol, each 250ml is a fraction, check and combine by TLC, combine 2-8 fractions, concentrate to dryness, dissolve in 50% methanol, use reversed phase silica gel ( RP-C18) column chromatography, eluting with 50% methanol, each fraction was 100ml, and combined by TLC, 14-18 fractions were combined, concentrated to dryness, methanol-water recrystallization, and 0.54 g of white needle crystals were obtained. It was identified as 7-hydroxy-4'-methoxyflavane (7-hydroxy-4'methoxy-flavane) by spectrum technique, mp: 134-135°C, HPLC98.4%. Fractions 20-27 were combined, concentrated to dryness, ...
Embodiment 2
[0081] 1. The preparation of Dracaena ethanol extract:
[0082] Take Dracaena glabrata, crush it, add ethanol to reflux for extraction twice, filter, recover ethanol, and dry it to obtain the ethanol extract of Dracaena glabrata.
[0083] 2. Separation and purification:
[0084] 1) Take 0.5 kg of ethanol extract of Dracaena glabrata, grind it, add 4 times of petroleum ether to extract for 3 days, filter, and filter the residue for later use;
[0085] 2) The resulting filter residue was refluxed with 10 times the amount of chloroform to extract 3 times, each time for 30 minutes, and the chloroform was recovered to obtain the chloroform extract;
[0086] 3) Dissolve the obtained chloroform extract in 60% methanol, perform macroporous resin column chromatography, and elute with 60% methanol, each 250ml is a fraction, TLC inspection and combination, and 2-8 fractions are combined;
[0087] 4) Concentrate the combined 2-8 fractions to dryness, dissolve with 50% methanol, and use ...
Embodiment 3
[0092] 1. The preparation of Dracaena ethanol extract:
[0093] Take Dracaena glabrata, crush it, add ethanol to reflux for extraction twice, filter, recover ethanol, and dry it to obtain the ethanol extract of Dracaena glabrata.
[0094] 2. Separation and purification:
[0095] 1) Take 0.5kg of the ethanol extract of Dracaena glabrata, grind it, add 8 times petroleum ether to extract for 1 day, filter, and filter the residue for later use;
[0096] 2) The resulting filter residue was refluxed and extracted 3 times with 6 times the amount of chloroform, each time for 30 minutes, and the chloroform was recovered to obtain the chloroform extract;
[0097] 3) Dissolve the obtained chloroform extract in 60% methanol, perform macroporous resin column chromatography, and elute with 60% methanol, each 250ml is a fraction, TLC inspection and combination, and 2-8 fractions are combined;
[0098] 4) Concentrate the combined 2-8 fractions to dryness, dissolve with 50% methanol, and use...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com