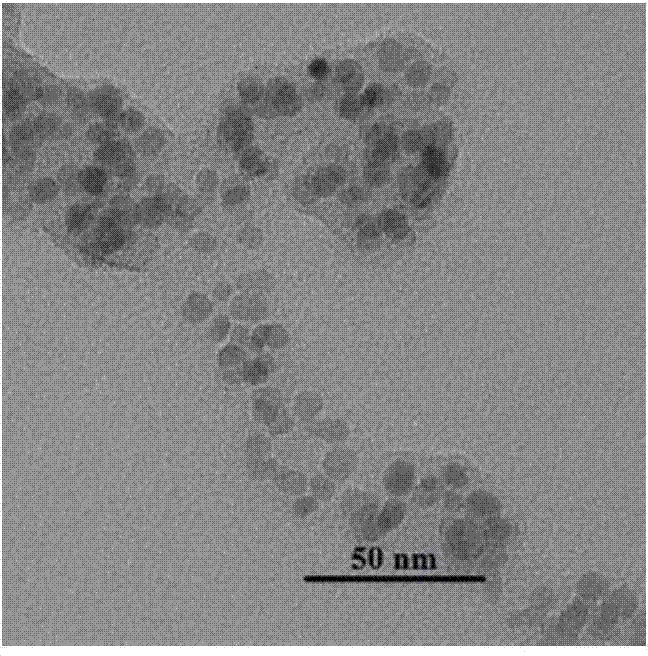
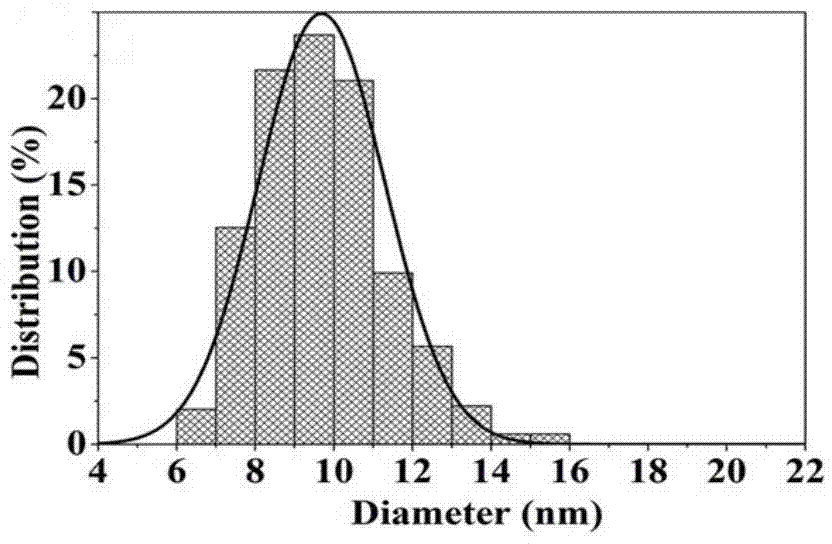
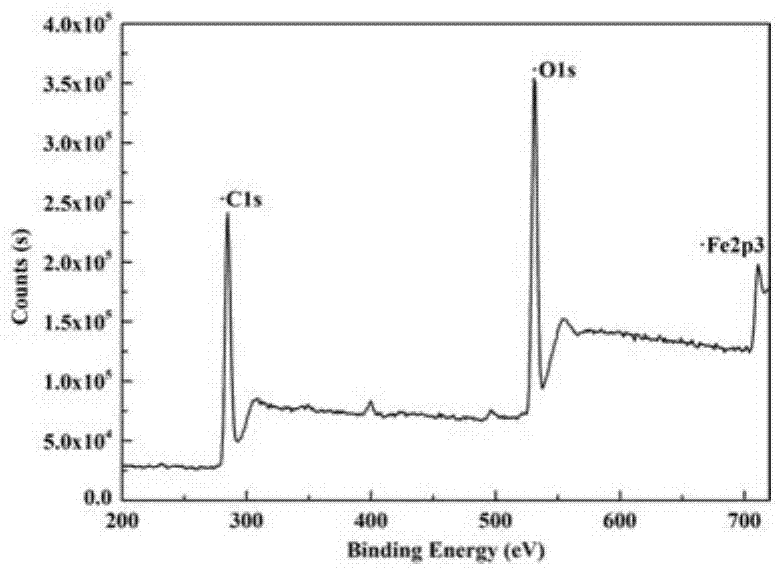
Carbon coated Fe3O4 composite material and preparation method thereof
A composite material, fe3o4 technology, applied in the field of carbon-coated Fe3O4 composite material and its preparation, can solve the problems of discontinuous preparation, time-consuming, complicated production process of composite material, etc., achieve continuous preparation, convenient and simple adjustment of preparation conditions, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) FeCl 3 ·6H 2 O, FeSO 4 ·7H 2 O was dissolved in deionized water to obtain solution 1, in which FeCl 3 and FeSO 4 The concentrations of NaOH and starch were 0.15mol / L and 0.075mol / L, respectively; NaOH and starch were dissolved in the same volume of deionized water to obtain solution 2, in which the concentrations of NaOH and starch were 0.6mol / L and 3.6mol / L, respectively. The concentration ratio of the amount of substance of each substance is Fe 3+ :Fe 2+ :OH - :starch=0.25:0.125:1:6.
[0022](2) Solution 1 and solution 2 obtained in step 1 are continuously input into the microreactor with the same flow rate 20ml / min through two different inlets of the microreactor respectively by a material pump, and react therein to generate Fe 3 o 4 At the same time, the reaction mixture is continuously exported from the outlet of the microreactor, and enters the spray pyrolysis reactor through the connecting pipeline, and is continuously fed with the inert carrier gas ...
Embodiment 2
[0026] (1) FeCl 3 ·6H 2 O, FeSO 4 ·7H 2 O was dissolved in deionized water to obtain solution 1, in which FeCl 3 and FeSO 4 The concentrations of NaOH and glucose were 0.15mol / L and 0.075mol / L, respectively; NaOH and glucose were dissolved in the same volume of deionized water to obtain solution 2, in which the concentrations of NaOH and glucose were 0.6mol / L and 3.6mol / L, respectively. The concentration ratio of the amount of substance of each substance is Fe 3+ :Fe 2+ :OH - :glucose=0.25:0.125:1:6.
[0027] (2) Solution 1 and solution 2 obtained in step 1 are continuously input into the microreactor with the same flow rate 20ml / min through two different inlets of the microreactor respectively by a material pump, and react therein to generate Fe 3 o 4 At the same time, the reaction mixture is continuously exported from the outlet of the microreactor, and enters the spray pyrolysis reactor through the connecting pipeline, and is continuously fed with the inert carrier...
Embodiment 3
[0030] (1) FeCl 3 ·6H 2 O, FeSO 4 ·7H 2 O was dissolved in deionized water to obtain solution 1, in which FeCl 3 and FeSO 4 The concentrations of NaOH and sucrose were 0.15mol / L and 0.075mol / L, respectively; NaOH and sucrose were dissolved in the same volume of deionized water to obtain solution 2, in which the concentrations of NaOH and sucrose were 0.6mol / L and 3.6mol / L, respectively. The concentration ratio of the amount of substance of each substance is Fe 3+ :Fe 2+ :OH - : sucrose=0.25:0.125:1:6.
[0031] (2) Solution 1 and solution 2 obtained in step 1 are continuously input into the microreactor with the same flow rate 20ml / min through two different inlets of the microreactor respectively by a material pump, and react therein to generate Fe 3 o 4 At the same time, the reaction mixture is continuously exported from the outlet of the microreactor, and enters the spray pyrolysis reactor through the connecting pipeline, and is continuously fed with the inert carrie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com