New application of malaria erythrocytic stage inactivated whole organism vaccine
A technology of erythrocyst and vaccine, which is applied in the field of inactivated whole worm vaccine during erythrocyst, can solve the problems that affect popularization and application, unacceptable, short activity maintenance time, etc., and achieve the effect of expanding the scope of use and preventing mutual transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of embodiment 1 inactivated whole worm vaccine
[0022] First resuscitate the Plasmodium NSM strains preserved in liquid nitrogen, then infect 8-10 Balb / c mice with the resuscitated NSM, and collect NSM parasites as parasite antigens on the 4th day and the 9th day after infection, respectively. The main process of the parasite antigen acquisition process is as follows: the blood components of the mouse are obtained by taking blood from the heart, and added to a centrifuge tube containing heparin to avoid coagulation; the above blood cells are washed 3 times with PBS with a pH value of 7.4 and a concentration of 0.1mol / L After heparin was removed, saponin with a final concentration of 0.5% was added to lyse red blood cells to release intracellular parasite antigens; the above components were washed with PBS three times to remove heme, and the protozoan cells were lysed by repeated freezing and thawing to obtain available Antigens for experiments; resuspend th...
Embodiment 2
[0024] Example 2 The protective effect of whole worm immune serum on erythrocyst
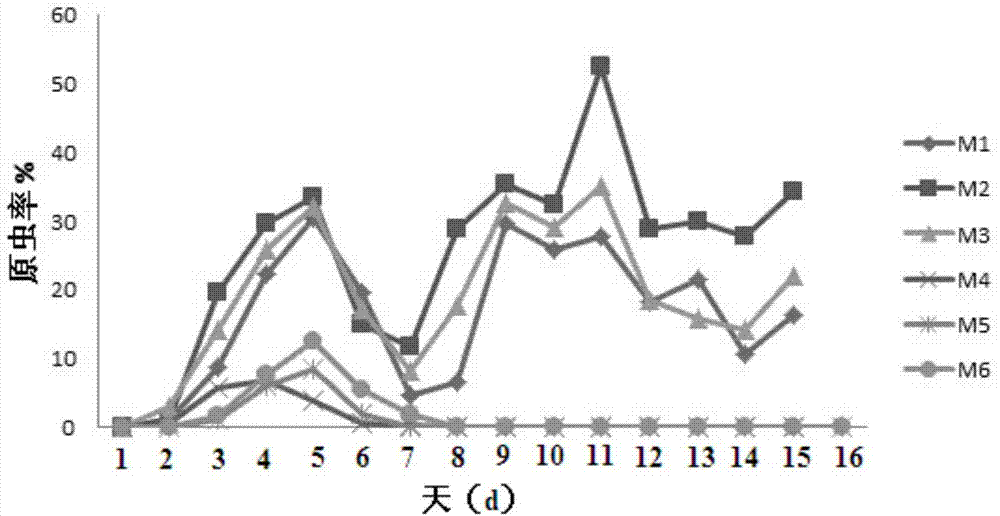
[0025] Resuscitate Plasmodium strain NSM stored in liquid nitrogen, press 1×10 6 The amount of protozoa / only infects 3 Balb / c mice after 3 times of immunizations in embodiment 1, and is compared with 3 normal Babl / c mice; Interval 24h gets tail tip blood and makes blood smear and counts protozoan blood disease. See the experimental results figure 1 , where M1-M3 are control mice, and M4-M6 are whole worm immunized mice. Depend on figure 1 It can be seen that Balb / c mice immunized with whole worms obtained a good protective effect against large doses of Plasmodium erythroplasma infection: the maximum parasite rate of immunized mice was reduced by more than 75%, and the clearance time of protozoa was shortened to 7 days. The vaccine contains all the antigens of the red inner stage of Plasmodium, so it can induce the host's immune system to produce a stronger immune response.
Embodiment 3
[0026] Example 3 Inactivated whole worm vaccine blocks mosquito transmission
[0027] First infect Anopheles mosquitoes with Plasmodium: Resuscitate NSM of Plasmodium strains preserved in liquid nitrogen at 1 x 10 6 6 Balb / c mice were infected at the rate of one per mouse, and the blood smear was used to evaluate the gametocytes of Plasmodium 4 days later, and the mice with the same gametocytes were selected for subsequent experiments.
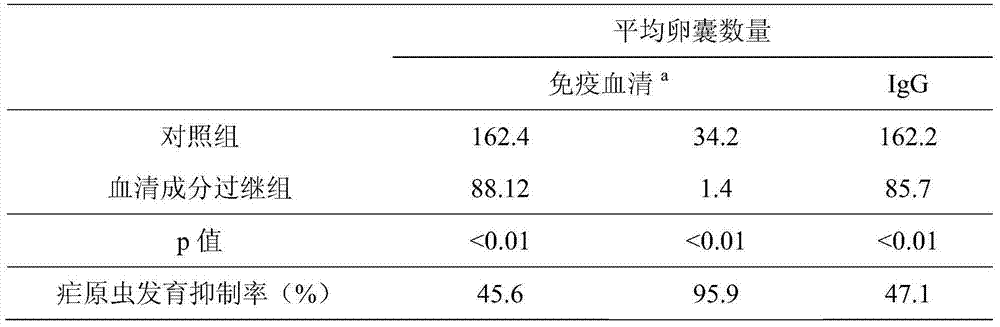
[0028] Observing the effect of inactivated holozoa vaccine immune serum in mosquito transmission blockage by adoptive holozoa immune serum; the specific IgG antibody in holozoa immune serum was obtained by protein-a protein affinity chromatography gel separation, Observing the role of specific antibodies in mosquito transmission blockage by IgG adoptive method (Table 1); injecting anti-CD4+T cell antibodies into mice to antagonize and inhibit the production of CD4+T cells to interferon (IFN-γ) In the way of mainly inflammatory factors, the ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com