Method used for preparing diethyl sulfate
A technology of diethyl sulfate and sulfuric acid, which is applied in the chemical industry, can solve the problems of low reuse value and pollution, and achieve the effects of fast evaporation speed, increased yield, and large liquid evaporation area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1) At room temperature, slowly drop 100 grams of 98% sulfuric acid into 92 grams of absolute ethanol, and stir for 30 minutes after the addition to obtain a pre-reaction mixture, which includes sulfuric acid, ethyl hydrogen sulfate, water, and ethanol ;
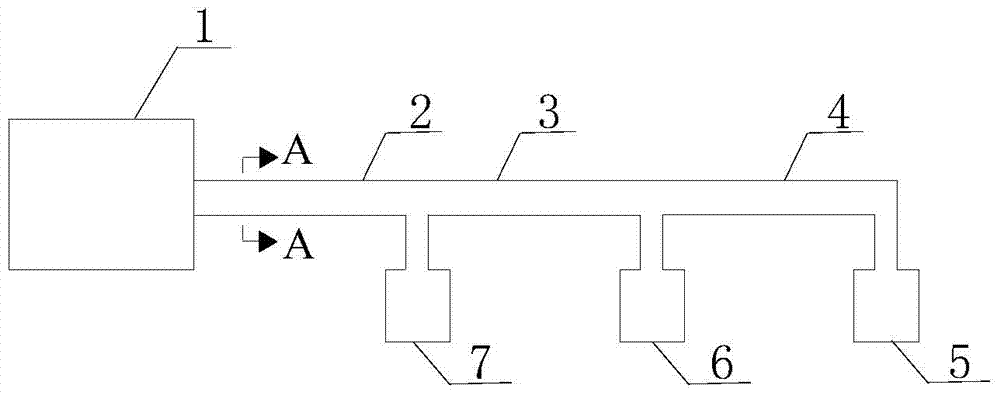
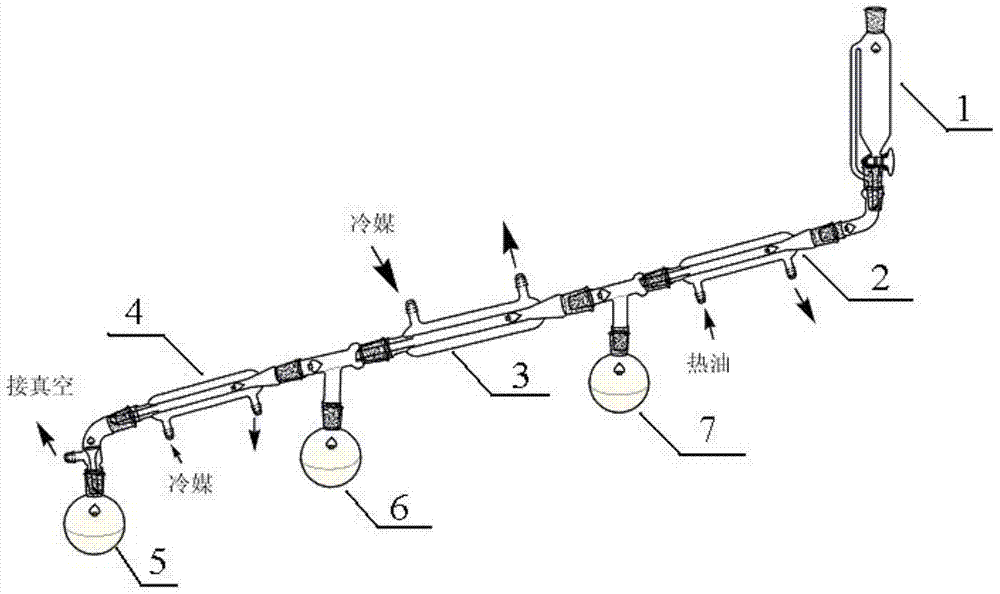
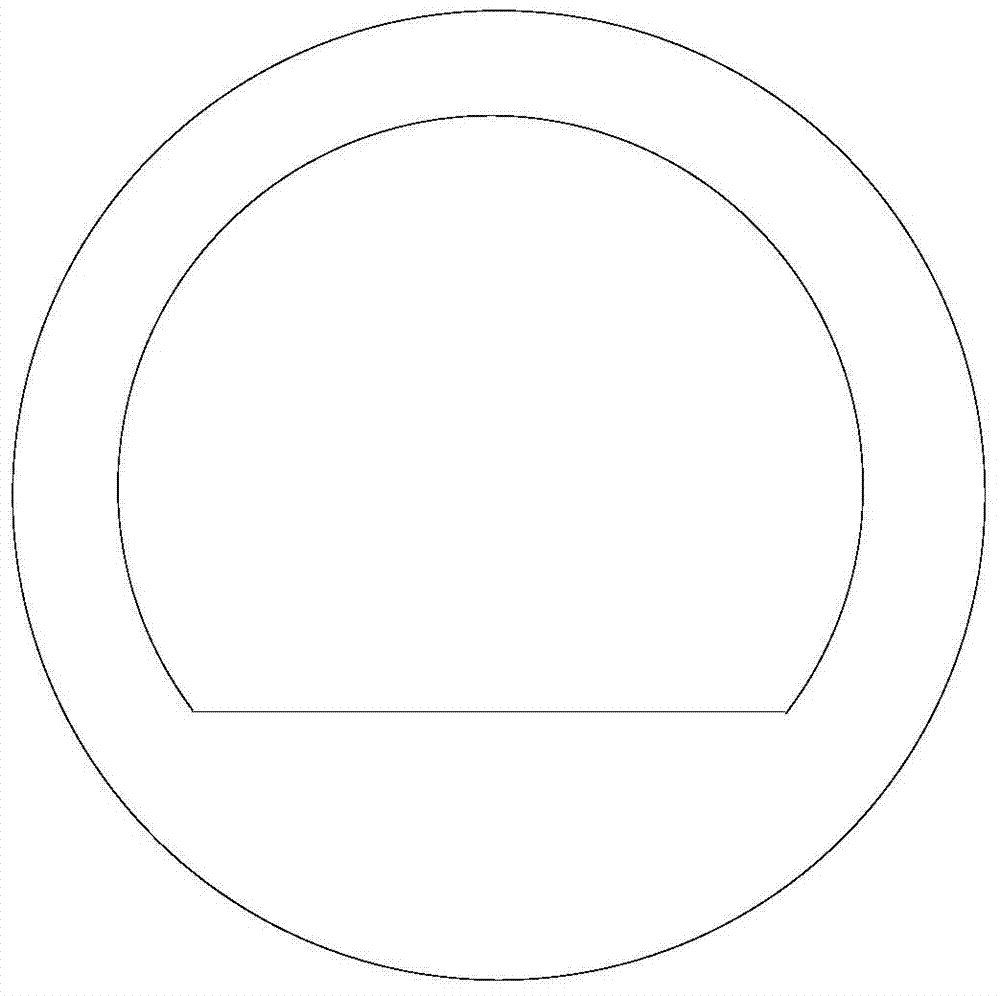
[0036] 2) figure 2 It is a process flow diagram for the preparation of diethyl sulfate. The pre-reaction liquid obtained in step 1) is added to the pre-reaction liquid dripping device 1, and enters the planar reaction section 2 in a laminar flow along the heating surface, and the planar reaction The A-A section of section 2 is a plane, such as image 3 As shown, the planar reaction section 2 maintains the temperature at 150-160°C, and the time for 1g of the pre-reaction liquid to flow through the heated planar reaction section is 0.5min. When the pre-reaction liquid flows through the planar reaction section 2, the pre-reaction Ethyl hydrogen sulfate in the liquid is continuously converted into diethyl sulfate, and d...
Embodiment 2
[0039] 1) 43 grams of ethylene are slowly passed into 75 grams of 98% sulfuric acid to obtain a reaction pre-reactant, which mainly contains diethyl sulfate, ethyl hydrogen sulfate and sulfuric acid in the reaction mixture;
[0040] 2) figure 2 It is a process flow diagram for the preparation of diethyl sulfate. The pre-reaction liquid obtained in step 1) is added to the pre-reaction liquid dripping device 1, and enters the planar reaction section 2 in a laminar flow along the heating surface, and the planar reaction The A-A section of section 2 is tubular, such as Figure 4 As shown, the planar reaction section 2 maintains the temperature at 145-155° C., and the time for 1 g of the pre-reaction solution to flow through the heated planar reaction section is 10 minutes. When the pre-reaction solution flows through the planar reaction section 2, the pre-reaction solution The ethyl hydrogen sulfate in the reaction system is continuously converted into diethyl sulfate, and dieth...
Embodiment 3
[0042] 1) At room temperature, slowly drop 100 grams of 98% sulfuric acid into 92 grams of absolute ethanol, and stir for 30 minutes after the addition to obtain a pre-reaction mixture, which includes sulfuric acid, ethyl hydrogen sulfate, water, and ethanol ;
[0043] 2) figure 2 It is a process flow diagram for the preparation of diethyl sulfate. The pre-reaction liquid obtained in step 1) is added to the pre-reaction liquid dripping device 1, and enters the planar reaction section 2 in a laminar flow along the heating surface, and the planar reaction The A-A section of section 2 is a plane, such as image 3 As shown, the planar reaction section 2 maintains the temperature at 130-150°C, and the time for 1g of the pre-reaction solution to flow through the heated planar reaction section is 4min. When the pre-reaction solution flows through the planar reaction section 2, the pre-reaction solution Ethyl hydrogen sulfate in the process is continuously converted into diethyl su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com