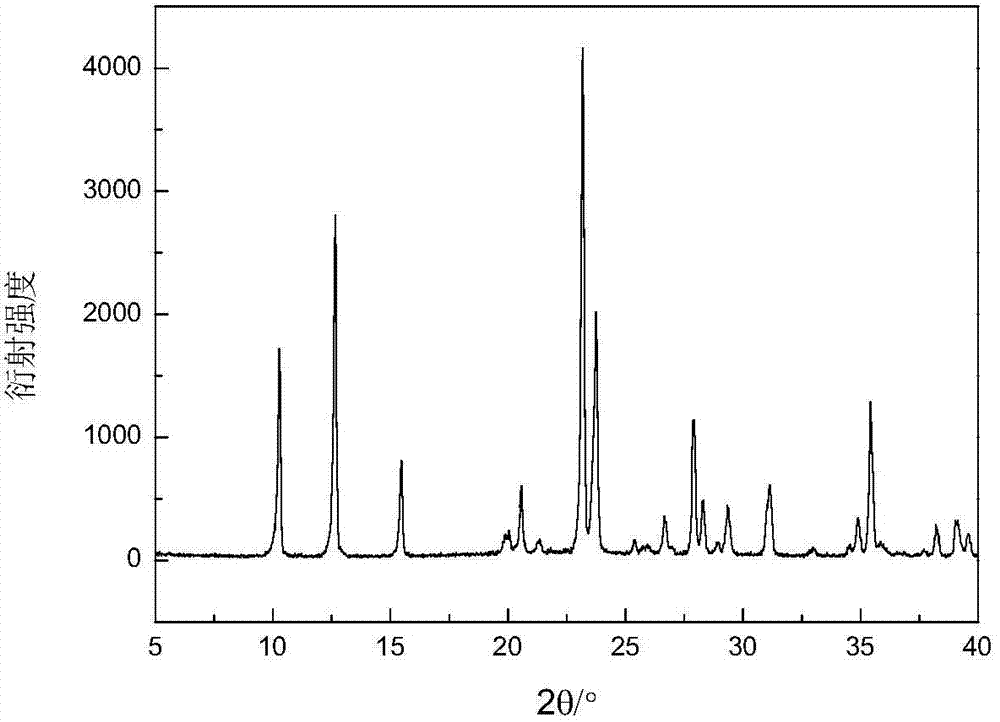
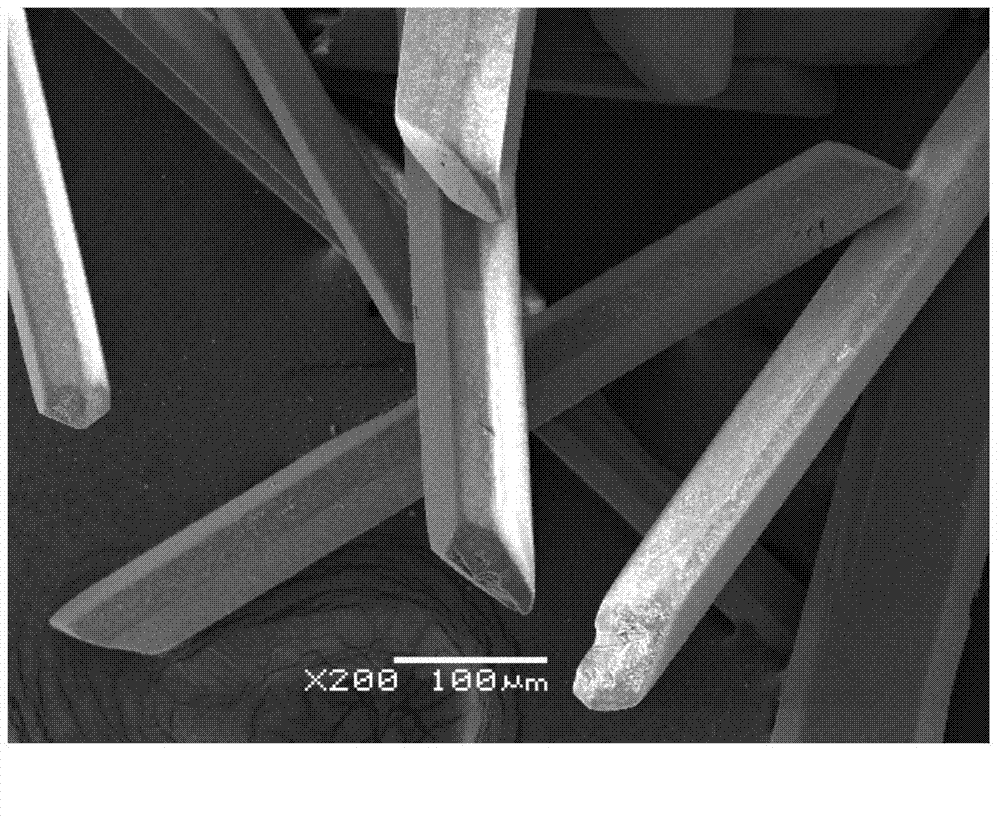
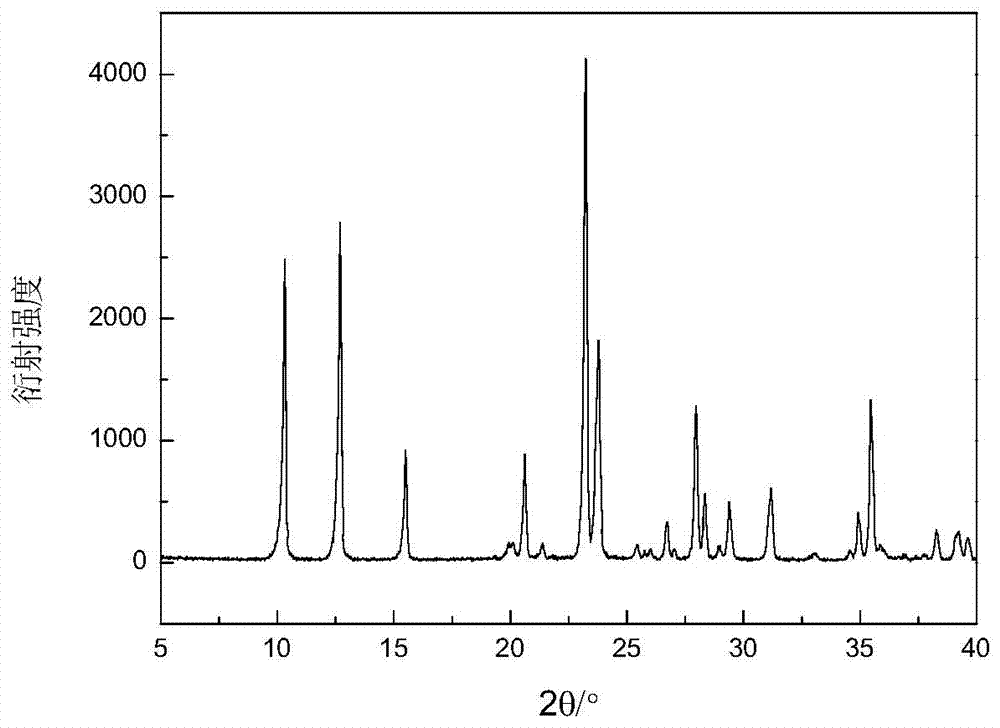
Method for preparing zinc phosphate compounds Zn(C3N2H4)HPO4 and Zn4P3O11(OH).3C3N2H4
A technology of 3C3N2H4 and C3N2H4, which is applied in the field of preparation of zinc phosphate compounds, can solve the problems of not further exploring the correlation of zinc phosphate compounds and the long time required for synthesis, and achieve uniform morphology, small crystal size and large specific surface area. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of zinc phosphate compound Zn(C 3 N 2 h 4 )HPO 4 The method includes the following steps:
[0047] (1) Add zinc acetate with a mass fraction of 99%, imidazole with a mass fraction of 99%, and phosphoric acid with a mass fraction of 85% into deionized water in sequence, and magnetically stir for 2 hours at a speed of 300r / min to obtain the initial mixing solution;
[0048] The mol ratio that the zinc acetate of 99% and the phosphoric acid of 85% is that the massfraction is 1:1.0; The mol ratio that the zinc acetate of 99% and the imidazole that massfraction is 99% is 1: 3.0; the mass fraction is 99% zinc acetate and the molar ratio of deionized water is 1:70;
[0049](2) Place the initial mixed solution obtained in step (1) in a special microwave reactor made of polytetrafluoroethylene, with a heating power of 400W, crystallize at 140°C for 90min, and cool naturally to room temperature to obtain Crystallize the product, then wash the crystallized produc...
Embodiment 2
[0052] Preparation of zinc phosphate compound Zn(C 3 N 2 h 4 )HPO 4 The method includes the following steps:
[0053] (1) Add zinc acetate with a mass fraction of 99%, imidazole with a mass fraction of 99%, and phosphoric acid with a mass fraction of 85% into deionized water in sequence, and magnetically stir for 2 hours at a speed of 300r / min to obtain the initial mixing solution;
[0054] The mol ratio that the zinc acetate of 99% and the phosphoric acid of 85% is that the massfraction is 1:1.0; The mol ratio that the zinc acetate of 99% and the imidazole that massfraction is 99% is 1: 3.0; the mass fraction is 99% zinc acetate and the molar ratio of deionized water is 1:70;
[0055] (2) Place the initial mixed solution obtained in step (1) in a special microwave reactor made of polytetrafluoroethylene, with a heating power of 600W, crystallize at 160°C for 30min, and cool naturally to room temperature to obtain Crystallize the product, then wash the crystallized produ...
Embodiment 3
[0058] Preparation of zinc phosphate compound Zn 4 P 3 o 11 (OH)·3C 3 N 2 h 4 The method includes the following steps:
[0059] (1) Add zinc acetate with a mass fraction of 99%, imidazole with a mass fraction of 99%, and phosphoric acid with a mass fraction of 85% into deionized water in sequence, and magnetically stir for 2 hours at a speed of 300r / min to obtain the initial mixing solution;
[0060] The mol ratio that the zinc acetate of 99% and the phosphoric acid of 85% is that the massfraction is 1:1.0; The mol ratio that the zinc acetate of 99% and the imidazole that massfraction is 99% is 1: 3.0; the mass fraction is 99% zinc acetate and the molar ratio of deionized water is 1:70;
[0061] (2) Place the initial mixed solution obtained in step (1) in a special microwave reactor made of polytetrafluoroethylene, with a heating power of 400W, crystallize at 180°C for 180min, and cool naturally to room temperature to obtain Crystallize the product, then wash the cryst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com