Basophilic granulocyte activation and degranulation identification method
A basophil identification method technology, applied in the field of allergic disease diagnosis, can solve the problems of basophil activation, allergen-specific basophil stimulation test cannot be carried out, etc., to achieve repeatable good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
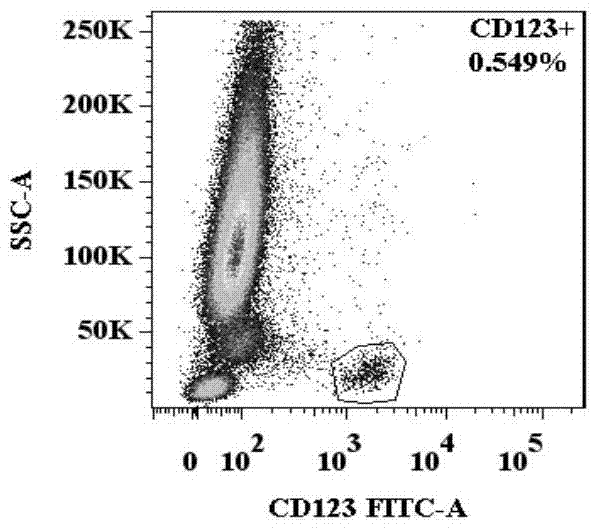
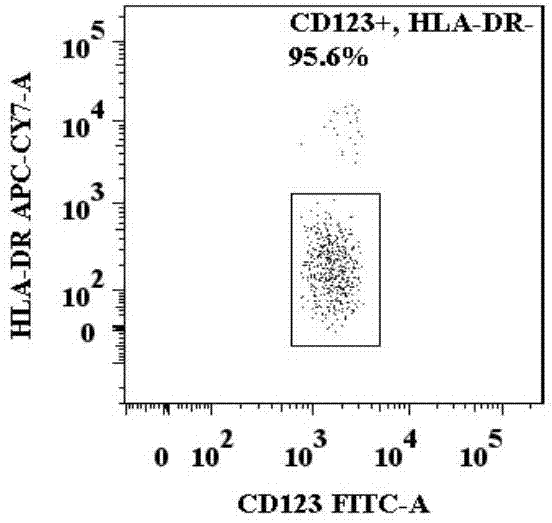
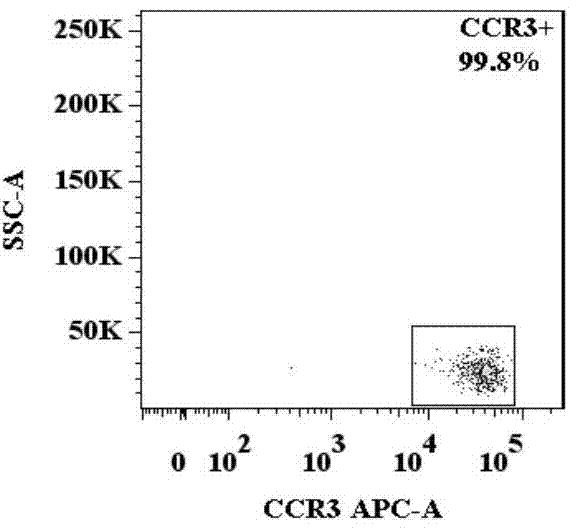
[0053] A method for identification of basophil activation and degranulation, such as Figure 1.1 , 1.2 , 1.3, 1.4, and 1.5, including the following steps:
[0054] (1) First, according to the ratio of 9:1, use 9 times of ultrapure water to dilute the concentrated erythrocyte lysate stock solution in the kit to the working state; Dilute the stock solution of phosphate buffered saline to working condition. Note: Red blood cell lysate is recommended to be prepared immediately;
[0055] (2) Number the test samples in sequence, and mark the flow sample loading tubes required for the test;
[0056] (3) Add the required antibody combination into each flow cytometry tube: anti-human CD123, anti-human CCR3, anti-human HLA-DR, anti-human CD203c and anti-human CD63 fluorescently labeled flow antibody combinations, such as FITC-labeled Anti-human CD123, APC-labeled anti-human CCR3, APC / Cy7-labeled anti-human HLA-DR, PE-labeled anti-human CD203c, PE / Cy7-labeled anti-human CD63, the amou...
Embodiment 2
[0066] A method for the identification of basophil activation, such as Figure 2.1 , 2.2 , 2.3, 2.4, including the following steps:
[0067] (1) First, according to the ratio of 9:1, use 9 times of ultrapure water to dilute the concentrated erythrocyte lysate stock solution in the kit to the working state; Dilute the stock solution of phosphate buffered saline to working condition. Note: Red blood cell lysate is recommended to be prepared immediately;
[0068] (2) Number the test samples in sequence, and mark the flow sample loading tubes required for the test;
[0069] (3) Add the required antibody combination into each flow cytometry sample tube: anti-human CD123, anti-human CCR3, anti-human HLA-DR, anti-human CD203c fluorescently labeled flow antibody combination, such as FITC-labeled anti-human CD123, APC-labeled anti-human CCR3, APC / Cy7-labeled anti-human HLA-DR, PE-labeled anti-human CD203c, the amount of each of the four antibodies is 5 μL per person to identify the...
Embodiment 3
[0079] A method for the identification of basophil degranulation, such as Figure 3.1 , 3.2 , 3.3, 3.4, including the following steps:
[0080] (1) First, according to the ratio of 9:1, use 9 times of ultrapure water to dilute the concentrated erythrocyte lysate stock solution in the kit to the working state; Dilute the stock solution of phosphate buffered saline to working condition. Note: It is recommended to prepare red blood cell lysate immediately after use.
[0081] (2) Number the test samples in a certain order, and mark the flow sample loading tubes required for the test;
[0082] (3) Add the required antibody combination into each flow cytometry sample tube: anti-human CD123, anti-human CCR3, anti-human HLA-DR, anti-human CD63 fluorescently labeled flow antibody combination, such as FITC-labeled anti-human CD123, APC-labeled anti-human CCR3, APC / Cy7-labeled anti-human HLA-DR, PE-labeled anti-human CD63, the amount of each of the four antibodies is 5 μL per person ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com