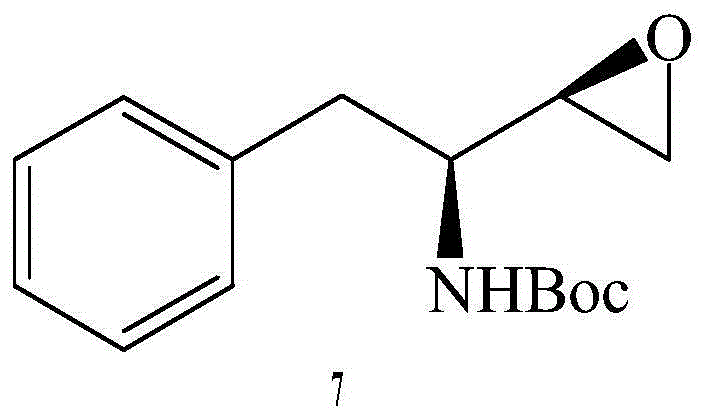
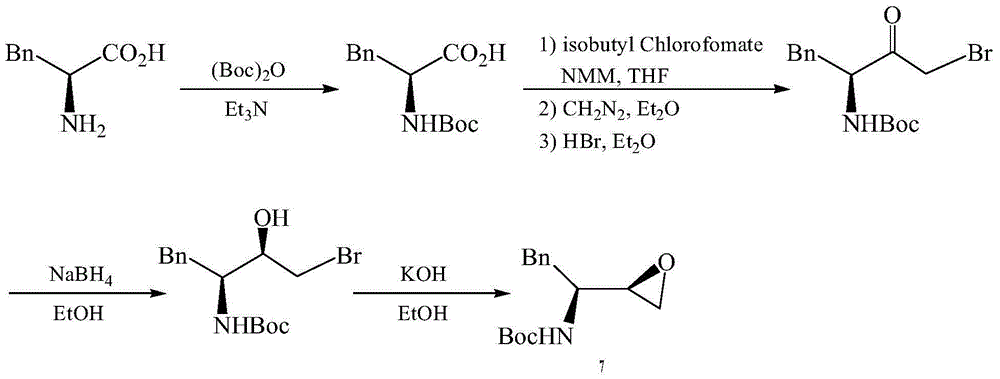
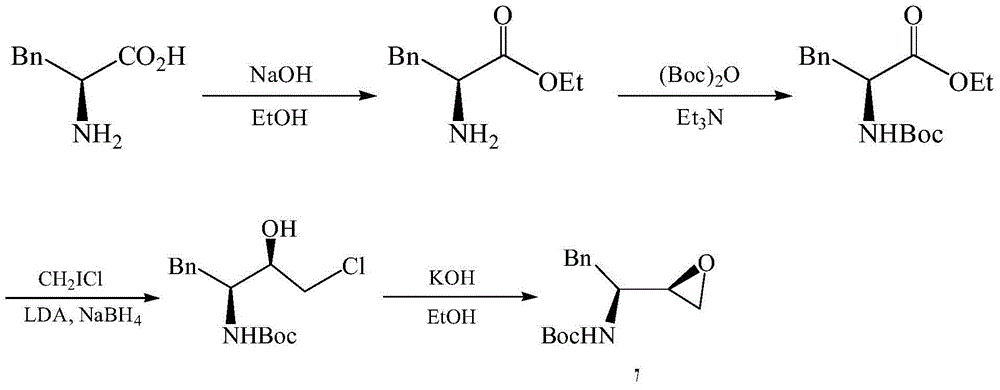
Preparation method for fosamprenir intermediate
A technique for the preparation of fosamprenavir and intermediates, which is applied in the field of preparation of fosamprenavir intermediates, -1,2-epoxy-3-tert-butoxycarbonylamino-4-phenylbutane, and can solve the problem of preparation The method solves problems such as complex process, low safety, and difficult separation, and achieves the effects of reasonable process, improved production efficiency, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) Adjust the pH of phenylacetonitrile 1 (60g, 0.512mol), potassium phosphate buffer (100ml), enzyme (20mg), and the mixture to 6, control the reaction temperature to 30°C, and stir for 12 hours. Then it was acylated with methoxymethylamine (31.3g, 0.512mol) at room temperature and stirred for 0.5h. After the reaction, extract with dichloromethane (2×250ml), dry, filter, concentrate under reduced pressure, column chromatography (ethyl acetate: hexane = 1:2) to give colorless oily liquid 2 (90.3g, 98.5% ). 1 H NMR (300MHz, CDCl 3 ):7.36-7.21(5H),3.78(s,2H),3.61(s,3H),3.2(s,3H). 13C NMR (75MHz, CDCl 3 ):172.5,135.1,129.4,128.6,126.9,61.4,39.5,32.3.
[0048] (2) Control the reaction temperature of N-methoxy-N-methyl-phenylacetamide 2 (90.3g, 0.504mol), bromovinyl Grignard reagent (66.3g, 0.51mol), tetrahydrofuran (250ml) mixture to 0 °C, stop the reaction after 1h. After the reaction, it was extracted with hexane (2×150ml), dried, filtered, concentrated under reduce...
Embodiment 2
[0055] (1) Adjust the pH of phenylacetonitrile 1 (60g, 0.512mol), potassium phosphate buffer (100ml), enzyme (20mg) and the mixture to 7, control the reaction temperature to 35°C, and stir for 13h. Then it was acylated with methoxymethylamine (46.8 g, 0.768 mol) at room temperature and stirred for 1 h. After the reaction, it was extracted with dichloromethane (2×250ml), dried, filtered, concentrated under reduced pressure, and column chromatography (ethyl acetate:hexane=1:2) gave colorless oily liquid 2 (90.5g, 98.7% ).
[0056] (2) Control the reaction temperature of N-methoxy-N-methyl-phenylacetamide 2 (90.5g, 0.505mol), bromovinyl Grignard reagent (98.4g, 0.757mol), tetrahydrofuran (250ml) mixture to 0 °C, stop the reaction after 1.5h. After the reaction, it was extracted with hexane (2×150ml), dried, filtered, concentrated under reduced pressure, and column chromatography (ethyl acetate:hexane=1:9) gave colorless oily liquid 3 (68.2g, 92.5%) .
[0057] (3) 4-phenyl-1-b...
Embodiment 3
[0062] (1) Adjust the pH of phenylacetonitrile 1 (60g, 0.512mol), potassium phosphate buffer (100ml), enzyme (20mg), and the mixture to 8, control the reaction temperature to 40°C, and stir for 14 hours. Then it was acylated with methoxymethylamine (62.5g, 1.024mol) at room temperature and stirred for 1.5h. After the reaction, it was extracted with dichloromethane (2×250ml), dried, filtered, concentrated under reduced pressure, column chromatography (ethyl acetate: hexane = 1:2) to obtain a colorless oily liquid 2 (89.9g, 98% ).
[0063](2) Control the reaction temperature of N-methoxy-N-methyl-phenylacetamide 2 (89.9g, 0.502mol), bromovinyl Grignard reagent (130.4g, 1.004mol), tetrahydrofuran (250ml) mixture to 0 °C, stop the reaction after 1.5h. After the reaction, it was extracted with hexane (2×150ml), dried, filtered, concentrated under reduced pressure, and column chromatography (ethyl acetate:hexane=1:9) gave colorless oily liquid 3 (68.9g, 94%) .
[0064] (3) 4-phe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com