Single-component visible light initiator and preparation method thereof
A photoinitiator and visible light technology, applied in the direction of organic chemistry, etc., can solve the problems of peculiar smell of the initiator system, low utilization rate of visible light, easy yellowing, etc., and achieves colorless film formation, easy deep curing, and wide spectral absorption range. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
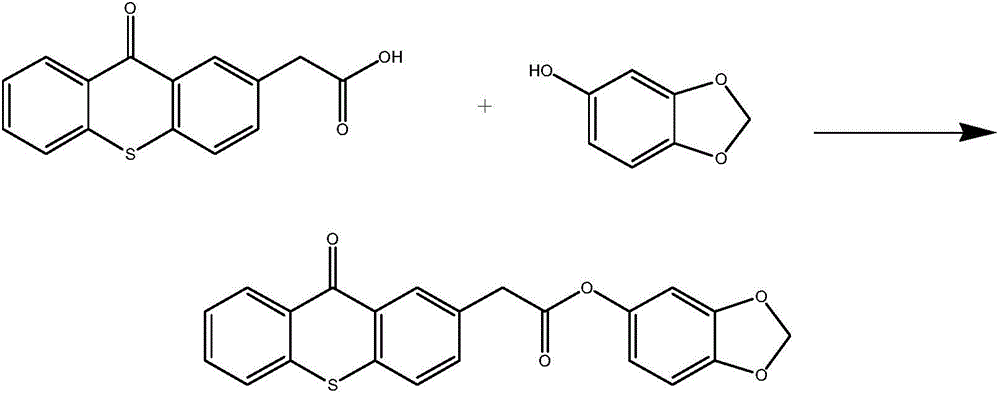
[0045] refer to figure 1 In the shown synthetic route, weigh 0.27g (1mmol) of 2-thioxanthone acetic acid and 0.138g (1mmol) of sesamol in a single-necked flask, then add 8mL of anhydrous toluene, and the dehydrating agent dicyclohexylcarbodiethylene Amine 0.619g (3mmol), catalyst 4-dimethylaminopyridine 0.365g (3mmol), after reflux reaction in the dark for 10 hours, spin the solvent, add 10mL of dichloromethane and 10mL of water for extraction, and dry the organic phase over anhydrous magnesium sulfate Overnight and filtered, the filtrate was concentrated and separated by column chromatography to obtain a colorless solid (eluent: ethyl acetate / petroleum ether=5 / 1), which was obtained after vacuum drying figure 1 Shown target product (yield 61%), the product is as follows through nuclear magnetic resonance spectrum determination structure:
[0046] 1 H NMR (400MHz, CDCl 3 )δ8.68-8.60 (m, 2H), 7.74-7.49 (m, 6H), 6.79-6.53 (m, 3H), 5.99 (s, 2H), 3.99 (s, 2H).
[0047] The ult...
Embodiment 2
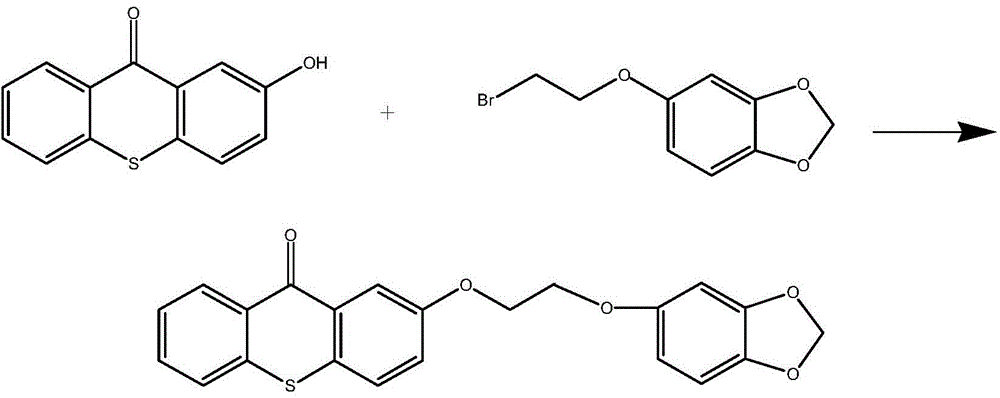
[0050] refer to figure 2In the synthetic route shown, weigh 0.228g (1mmol) of 2-hydroxythioxanthone and 0.186g of 5-(2-bromoethyl)-1,3-benzodioxole in a single-necked flask (1mmol), then add 8mL of anhydrous toluene, reflux reaction in the dark for 10 hours, spin to dry the solvent, add 10mL of dichloromethane and 10mL of water for extraction, the organic phase is dried overnight with anhydrous magnesium sulfate and filtered, and the filtrate is concentrated and passed through the column Chromatographic separation gave a colorless solid (eluent: ethyl acetate / petroleum ether=10 / 1), which was obtained after vacuum drying figure 2 Shown target product (yield 58%), the product is as follows through nuclear magnetic resonance spectrum determination structure:
[0051] 1 H NMR (400 MHz, DMSO) δ 10.18 (s, 1H), 8.46 (m, 1H), 7.90-7.52 (m, 5H), 7.32-7.23 (m, 1H).
[0052] The ultraviolet-visible light absorption spectrum figure of gained photoinitiator is shown in Figure 4 ,Dep...
Embodiment 3
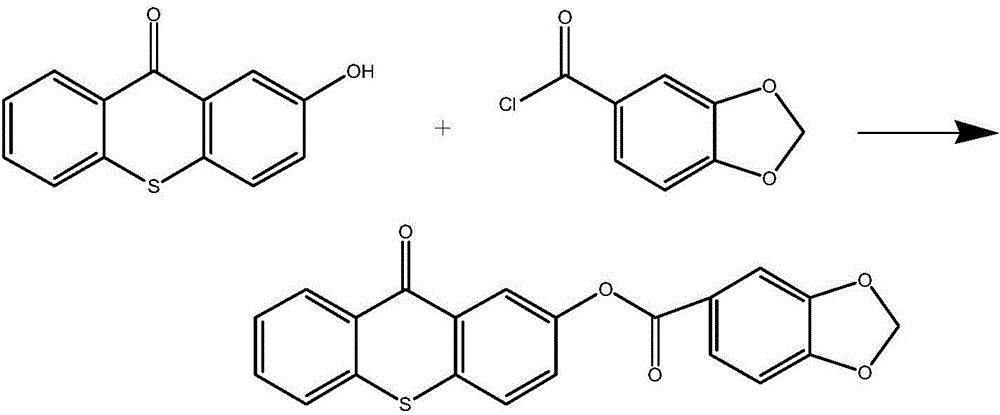
[0054] refer to image 3 In the synthetic route shown, add 0.228g (1mmol) of 2-hydroxythioxanthone and 0.184g (1mmol) of piperonyl chloride into a single-necked flask, then add 8mL of anhydrous toluene, and then reflux the reaction in the dark for 10 hours, then spin to dry the solvent , adding dichloromethane 10mL and saturated sodium bicarbonate solution 10mL for extraction three times, the organic phase was dried overnight over anhydrous magnesium sulfate and filtered, the filtrate was concentrated and separated by column chromatography to obtain a colorless solid (eluent: ethyl acetate / petroleum ether=5 / 1), obtain photoinitiator of the present invention (productive rate 42%) after vacuum-drying, product determines structure as follows through nuclear magnetic resonance spectrum:
[0055] 1 H NMR (400MHz, CDCl 3 )δ8.64-8.60 (m, 1H), 8.42 (d, J = 2.5Hz, 1H), 7.85 (m, 1H), 7.64-7.49 (m, 6H), 6.92 (d, J = 8.2Hz, 1H ), 6.09(s,2H).
[0056] The ultraviolet-visible light abs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com