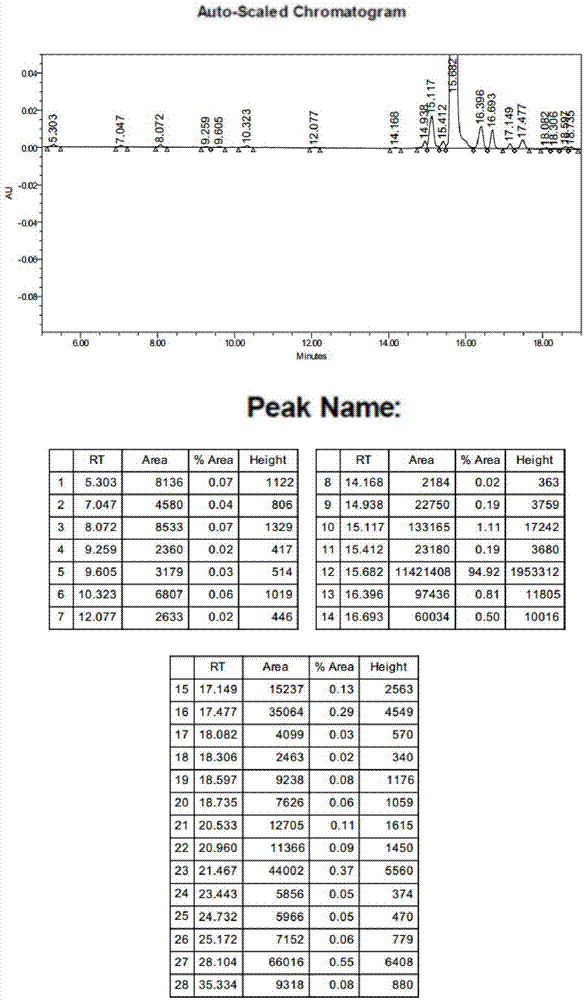
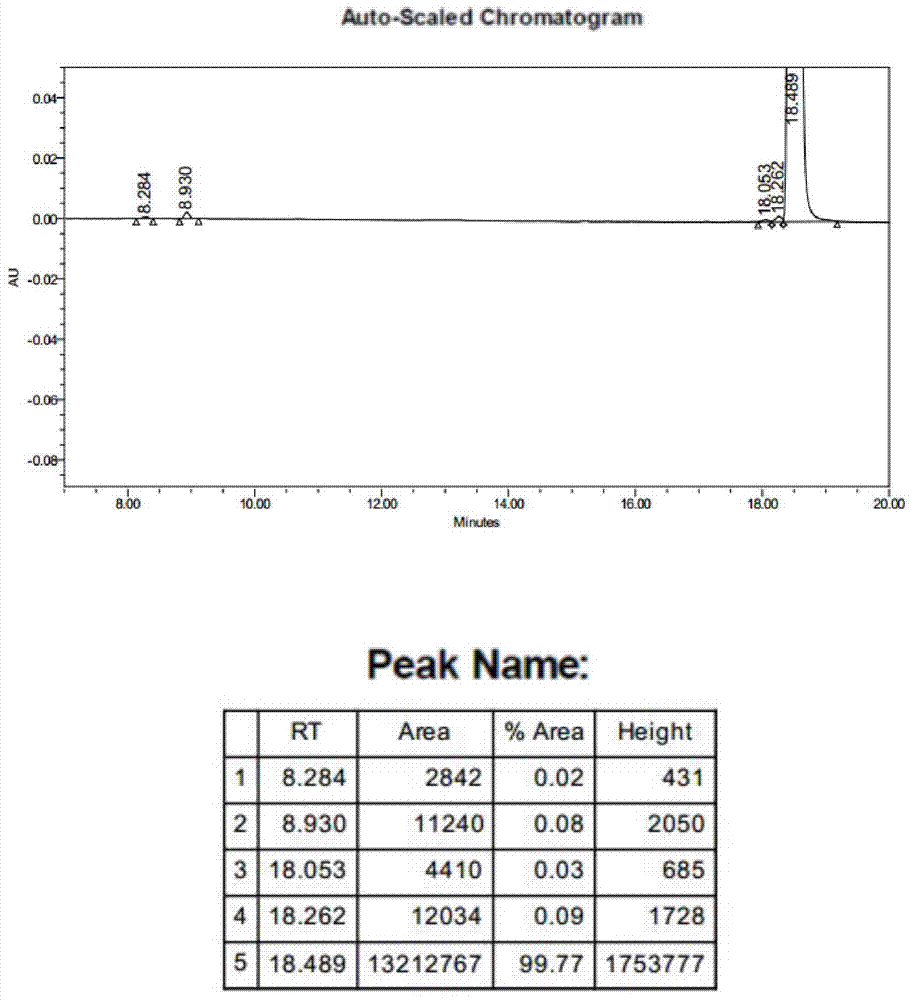
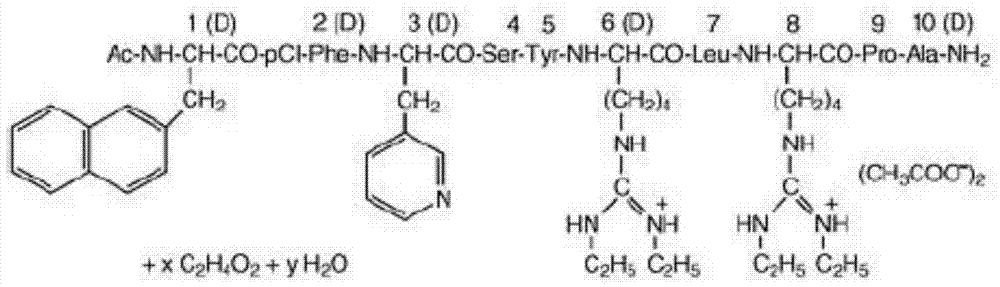
Ganirelix acetate preparation method
A technology of Ganirelix and acetic acid, which is applied in the field of drug synthesis, can solve problems such as increased side reactions, non-reaction, and large environmental pollution, and achieve the effects of suppressing side reactions, increasing yield, and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Synthesis of Ganyrelix Precursor I
[0039] 1. Preparation of Fmoc-D-Ala-Rink Amide resin
[0040] 40 g of Rink Amide resin with a substitution degree of 0.5 mmol / g was loaded into a solid-phase reaction column, washed twice with DMF, swollen with DMF for 30 minutes, deprotected twice for 10 minutes and 15 minutes respectively, washed with DMF for 6 times, Ninhydrin test was positive.
[0041] Fmoc-D-Ala-OH (18.68g, 60mmol) and HOBt (8.52g, 63mmol) were dissolved in 150ml DMF, and DIC (9.9ml, 63mmol) was added to activate for 5 minutes under ice-cooling conditions, and the activated solution was added Into the solid-phase reaction column, nitrogen stirring reaction for 2 hours, ninhydrin detection was negative. Drain the reaction solution, wash with DMF three times, deprotect DBLK twice for 5 minutes and 7 minutes respectively, wash with DMF for 6 times, and the ninhydrin test is positive.
[0042] 2. Coupling of other amino acids of the Fomc protecting gr...
Embodiment 2
[0049] Embodiment 2: Synthesis of Ganirelix Crude Product
[0050] The ganirelix precursor I (3.1 g, 2 mmol) obtained in Example 1 and ethylaminoethyleneimino methanesulfonic acid (0.72 g, 4 mmol) were dissolved in 20 ml H 2 In O, 10% NaOH was slowly added dropwise at room temperature to adjust the pH to 7.5, mechanically stirred for 8 hours, and 10% NaOH was added during the reaction to maintain the pH at 7.5, and the product purity in the reaction solution was 35%.
Embodiment 3
[0051] Embodiment 3: the synthesis of Ganirelix crude product
[0052] The ganirelix precursor I (3.1 g, 2 mmol) obtained in Example 1 and ethylaminoethyleneimino methanesulfonic acid (0.72 g, 4 mmol) were dissolved in 20 ml H 2 In O, 10% NaOH was slowly added dropwise at room temperature to adjust the pH to 9.0, and the reaction was carried out with mechanical stirring for 8 hours. During the reaction, 10% NaOH was added to maintain the pH at 9.0, and the product purity in the reaction solution was 41%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com