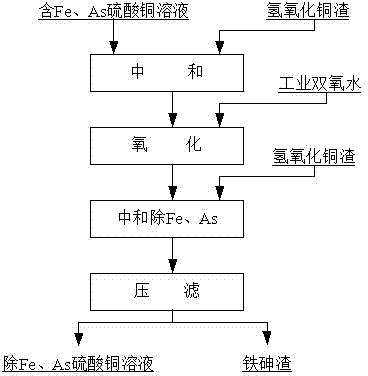
Method for removing iron and arsenic in copper sulfate solution
A technology of copper sulfate solution and copper sulfate, which is applied in the field of removing arsenic and iron from copper sulfate solution, can solve problems such as narrow application range, and achieve the effect of short process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) take 1m 3 Copper sulfate solution is put into stirring tank, and composition is: Cu 69.36g / L, full Fe 4.50g / L (wherein Fe 2+ accounted for 30%), all As 2.61g / L (of which As 3+ 70%), H 2 SO 4 23.87g / L, start stirring and add copper hydroxide slag to adjust the pH of the solution to 1.0.
[0032] 2) Mole ratio H 2 o 2 :( 2+ +As 3+ )=2.0:1, add 4.5L of industrial-grade hydrogen peroxide, use a connecting pipe to add industrial-grade hydrogen peroxide to the bottom of the stirring tank, add slowly, complete the adding process in 10 minutes, and control the oxidation time to 2.0h.
[0033] 3) Add copper hydroxide slag again, the reaction time is 3.0 h, and the end point pH is 3.5.
[0034] 4) Separation of liquid and solid, washing of the filter cake to obtain 1.2m copper sulfate solution for removing iron and arsenic 3 (including filter cake washing water), the composition is: Cu 70.08g / L, Fe 0.03g / L, As 0.09g / L. The iron removal rate is 99.20%, and the arsenic...
Embodiment 2
[0037] 1) take 1m 3 Copper sulfate solution, composition is: Cu 53.25g / L, full Fe 7.43g / L (wherein Fe 2+ accounted for 40%), all As 6.64g / L (of which As 3+ 55%), H 2 SO 4 26.54g / L, start stirring and add copper hydroxide slag to adjust the pH of the solution to 1.0.
[0038] 2) Mole ratio H 2 o 2 :( 2+ +As 3+ )=3.0:1, add 9.45L of industrial-grade hydrogen peroxide, use a connecting pipe to add industrial-grade hydrogen peroxide to the bottom of the stirring tank, add slowly, complete the adding process in 10 minutes, and control the oxidation time to 1.5h.
[0039] 3) Add copper hydroxide slag again, the reaction time is 2.5 h, and the end point pH is 3.0.
[0040] 4) Separation of liquid and solid, washing of the filter cake to obtain 1.3m copper sulfate solution for removing iron and arsenic 3 (including filter cake washing water), the composition is: Cu 53.96g / L, Fe 0.01g / L, As 0.07g / L. The iron removal rate is 99.80%, and the arsenic removal rate is 98.63%.
[...
Embodiment 3
[0043] 1) take 1m 3 Copper sulfate solution, composition is: Cu 63.32g / L, full Fe 3.37g / L (wherein Fe 2+ accounted for 25%), all As 2.66g / L (of which As 3+ 40%), H 2 SO 4 32.57g / L, start stirring and add copper hydroxide slag to adjust the pH of the solution to 1.5.
[0044] 2) Mole ratio H 2 o 2 :( 2+ +As 3+ )=2.5:1, add 2.26L of industrial-grade hydrogen peroxide, use a connecting pipe to add industrial-grade hydrogen peroxide to the bottom of the stirring tank, add slowly, complete the adding process in 10 minutes, and control the oxidation time to 0.5h.
[0045] 3) Add copper hydroxide slag again, the reaction time is 1.5 h, and the end point pH is 2.5.
[0046] 4) Separation of liquid and solid, washing of the filter cake to obtain 1.2m copper sulfate solution for removing iron and arsenic 3 (including filter cake washing water), the composition is: Cu 67.85g / L, Fe 0.02g / L, As 0.07g / L. The iron removal rate is 99.29%, and the arsenic removal rate is 96.84%.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com