Water treatment method for removing trihalomethane in drinking water by utilizing UV (ultraviolet)/H2O2
A technology for trihalomethane and drinking water, applied in the field of water treatment, can solve problems such as high trihalomethane, and achieve the effects of simple reaction device, no secondary pollution and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
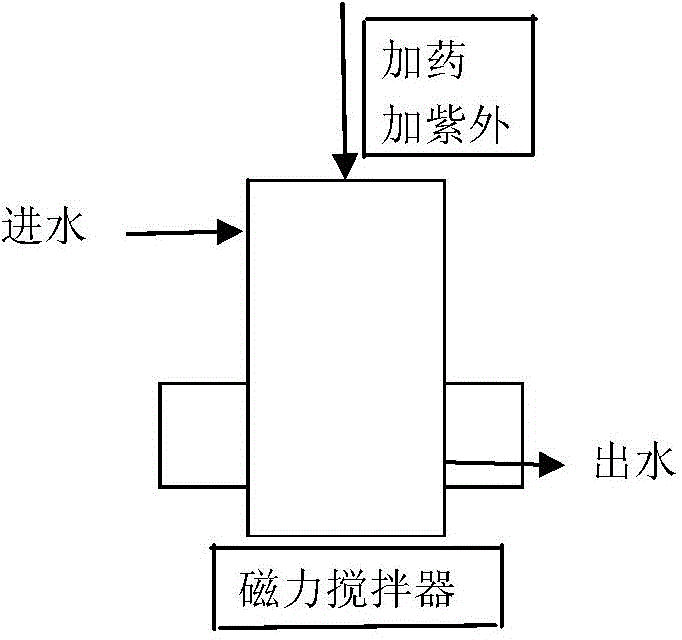
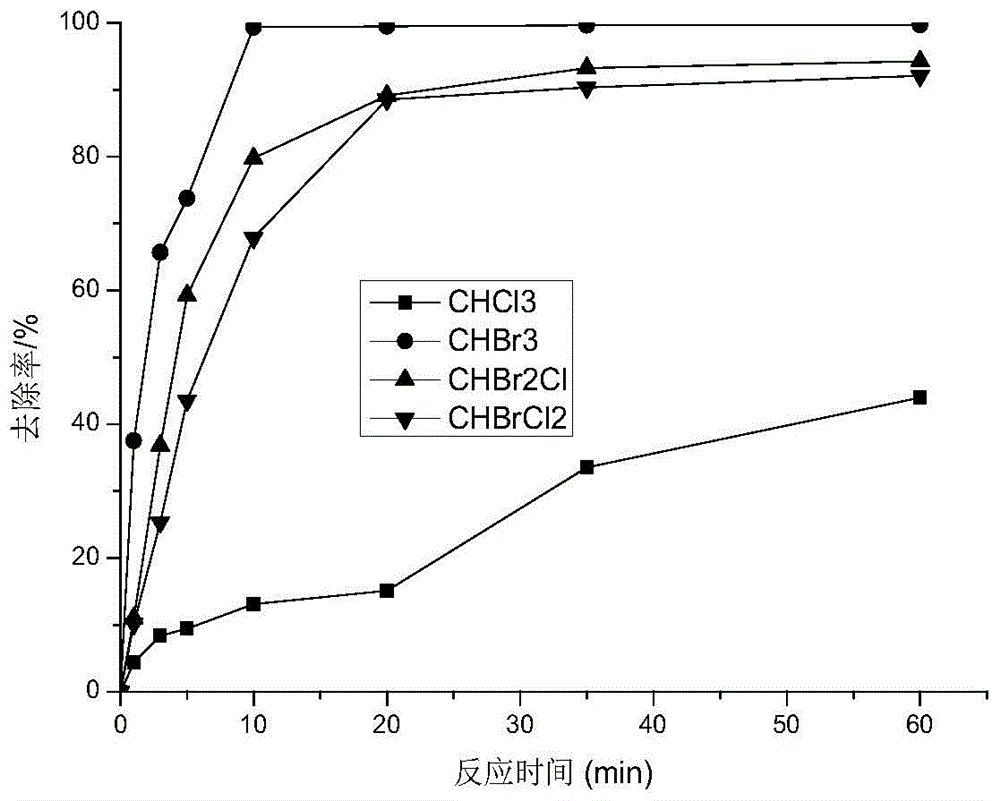
[0022] Embodiment 1: Take 1 L of trihalomethane mixed solution prepared with drinking water in figure 1 In the reaction device shown, add hydrogen peroxide to the reaction device, the dosage of hydrogen peroxide is 10mmol / L, then put the 21W ultraviolet lamp with quartz sleeve that has been luminescent stably into the reaction solution, and turn on the magnetic stirring at the same time Take a certain volume of reaction solution from the sampling port at 0min, 1min, 3min, 5min, 10min, 20min, 35min and 60min, respectively, and analyze the concentration of trihalomethane components by headspace gas chromatography. Such as figure 2 Shown, time has bigger influence on trihalomethane removal rate, and tribromomethane removal rate reaches 99.71% in 10min, and removal rate changes little afterwards, and the removal rate of dibromochloromethane and monobromodichloromethane in 20min 94.28% and 92.06%, there is no obvious change in the removal rate after that, but the removal rate of ...
Embodiment 2
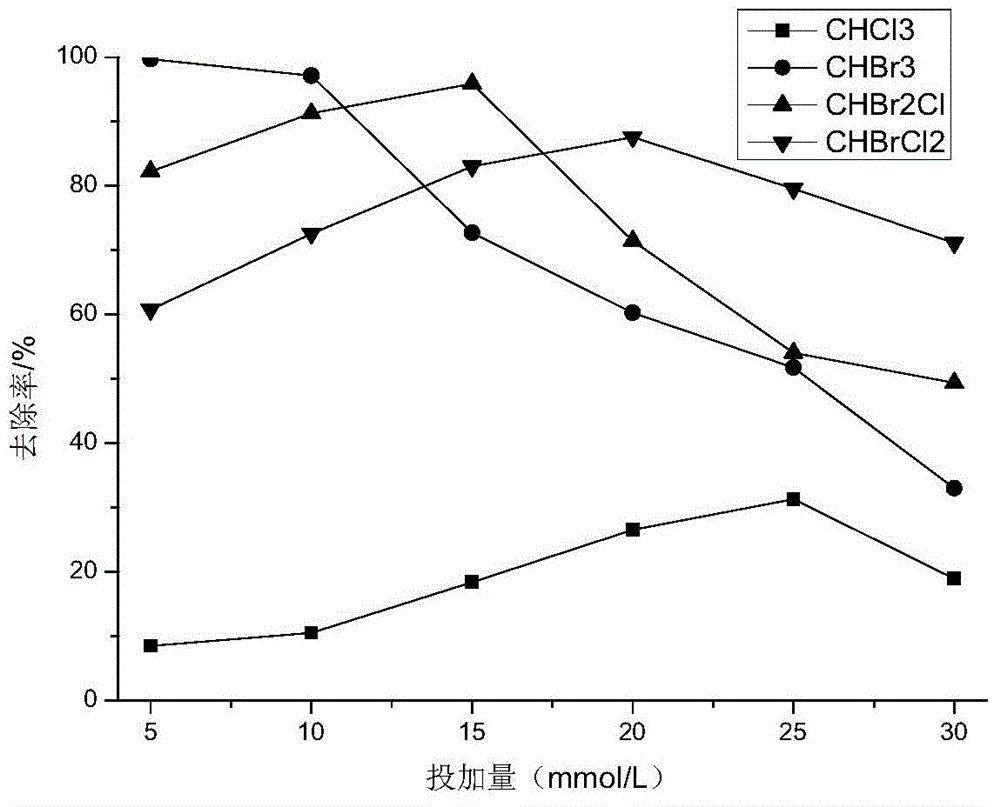
[0023] Example 2: Take 1L of the trihalomethane mixed solution prepared with drinking water in the reaction device, add hydrogen peroxide to the reaction device, the dosage of hydrogen peroxide is 5-30mmol / L, and then the luminescence has stabilized Put the 21W ultraviolet lamp with a quartz sleeve into the reaction solution, and turn on the magnetic stirrer at the same time. After reacting for 20 minutes, take a certain volume of the reaction solution from the sampling port, and use headspace gas chromatography to analyze the concentration of trihalomethane components. Such as image 3 As shown, the removal rate of each component of trihalomethane increases firstly and then decreases with the dosage of hydrogen peroxide. When the hydrogen dosage is 15mmol / L, the removal rate of dibromochloromethane reaches the maximum, which is 95.90%.
Embodiment 3
[0024] Embodiment 3: Take 1L of trihalomethane mixed solution prepared with domestic drinking water in the reaction device, add hydrogen peroxide to the reaction device, the dosage of hydrogen peroxide is 15mmol / L, and then put the luminescent stable band Put the ultraviolet lamp of the quartz sleeve into the reaction solution. The power of the ultraviolet lamp is 12-75W. At the same time, turn on the magnetic stirrer. After 20 minutes of reaction, take a certain volume of the reaction solution from the sampling port, and use headspace gas chromatography to analyze the trihalomethanes. component concentration. Such as Figure 4 As shown, the removal rate of each component of trihalomethane gradually increased. When the power of the ultraviolet lamp was 12W, 21W, 40W and 75W, the removal rate of trichloromethane was 13.58%, 25.06%, 32.36% and 41.41% respectively in 20 minutes. Three The removal rates of bromomethane were 84.60%, 98.42%, 99.58% and 99.61%, respectively, the rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com