Cheap low-temperature SCR catalyst with sulfur resistance and preparation method thereof
A technology of SCR catalyst and sulfur resistance, which is applied in the field of low-temperature SCR catalyst and preparation, can solve the problem of high cost of nitrate, achieve the effect of increasing catalytic activity, simple method, and improving dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
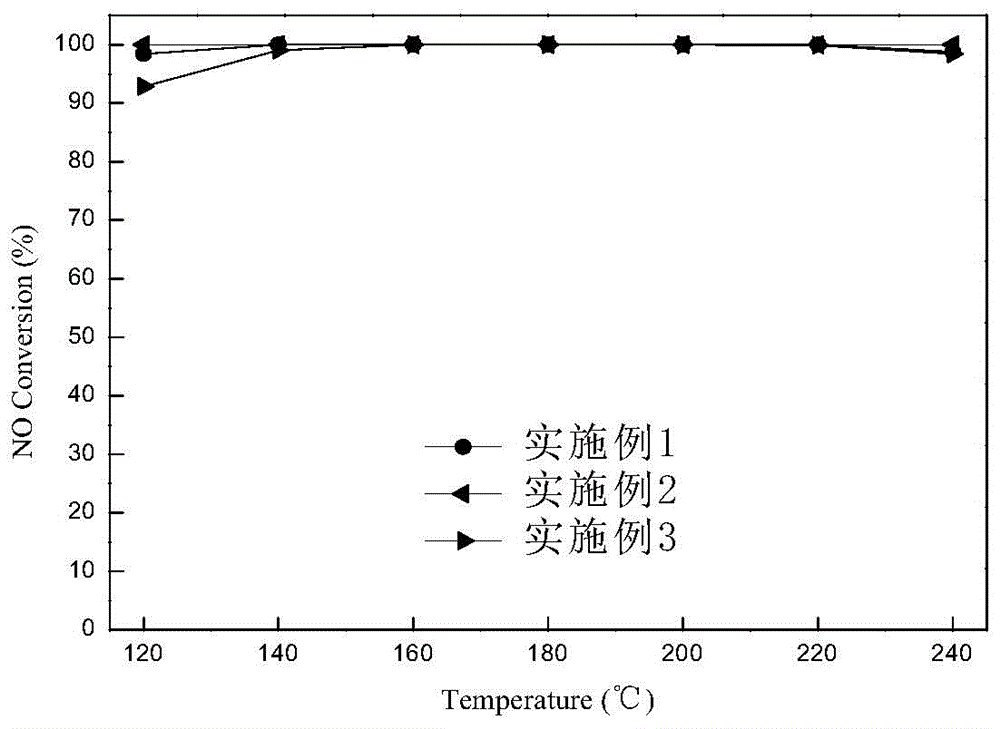
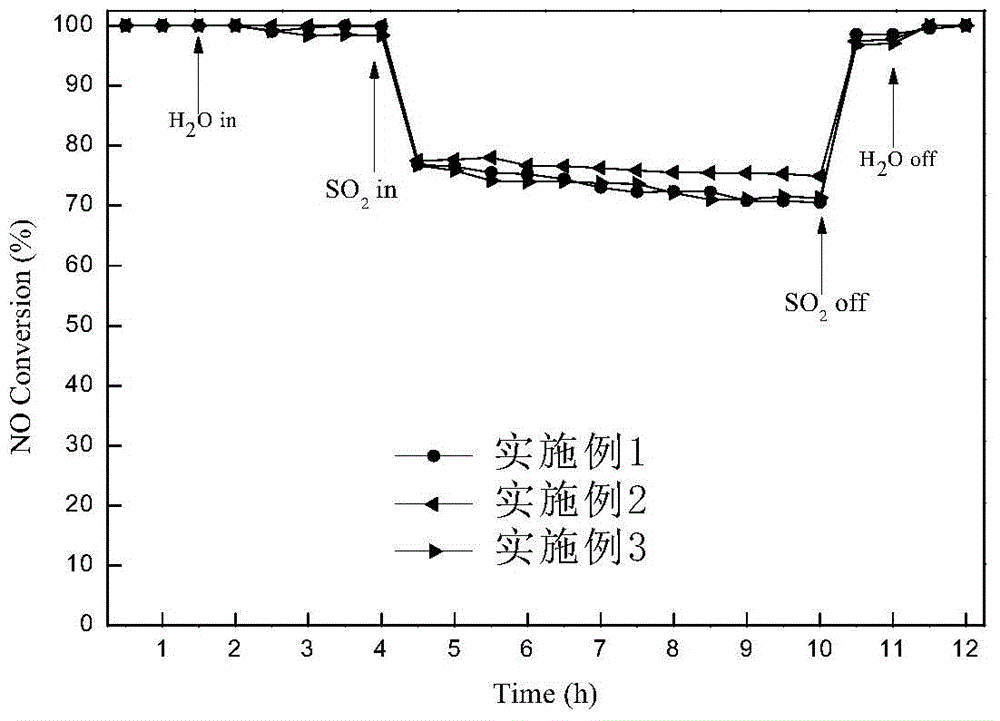
Embodiment 1
[0027] Catalyst preparation: weigh a certain amount of Al(NO 3 ) 3 Powder, add appropriate amount of deionized water to make 2.5mol / L Al(NO 3 ) 3 Solution, under vigorous stirring, slowly add 25% NH 3 ·H 2 O solution, until pH = 8-9, stop the dropwise addition, continue to stir for 3 hours, let stand for aging for 1 hour, centrifuge, and dry at 80°C for 12 hours to obtain Al(OH) 3 solid. Measure a certain amount of Mn (NO 3 ) 2 solution and Ce(NO 3 ) 2 solid, add a small amount of deionized water to make Ce(NO 3 ) 2 The solid dissolves and the resulting Al(OH) 3 The solid was impregnated with Mn(NO 3 ) 2 and Ce(NO 3 ) 2 in the mixed solution for 12h, and then the impregnated product was dried in a water bath at 80°C for 12h, and then placed in an oven at 110°C for 2h to obtain a black solid. Then put the black solid in a muffle furnace for calcination at 500°C for 6 hours, grind it, pass through a 40-60 mesh sieve, and obtain the catalyst Mn-Ce(0.02) / γ-Al 2 o ...
Embodiment 2
[0031] Catalyst preparation: weigh a certain amount of Al(NO 3 ) 3 Powder, add appropriate amount of deionized water to make 2.5mol / L Al(NO 3 ) 3 Solution, under vigorous stirring, slowly add 25% NH 3 ·H 2 O solution, until pH = 8-9, stop the dropwise addition, continue to stir for 3 hours, let stand for aging for 1 hour, centrifuge, and dry at 80°C for 12 hours to obtain Al(OH) 3 solid. Measure a certain amount of Mn (NO 3 ) 2 solution and Ce(NO 3 ) 2 solid, add a small amount of deionized water to make Ce(NO 3 ) 2 The solid dissolves and the resulting Al(OH) 3 The solid was impregnated with Mn(NO 3 ) 2 and Ce(NO 3 ) 2 in the mixed solution for 12h, and then the impregnated product was dried in a water bath at 80°C for 12h, and then placed in an oven at 110°C for 2h to obtain a black solid. Then put the black solid in a muffle furnace for calcination at 500°C for 6 hours, grind it, pass through a 40-60 mesh sieve, and obtain the catalyst Mn-Ce(0.12) / γ-Al 2 o ...
Embodiment 3
[0035] Catalyst preparation: weigh a certain amount of Al(NO 3 ) 3 Powder, add appropriate amount of deionized water to make 2.5mol / L Al(NO 3 ) 3 Solution, under vigorous stirring, slowly add 25% NH 3 ·H 2 O solution, until pH = 8-9, stop the dropwise addition, continue to stir for 3 hours, let stand for aging for 1 hour, centrifuge, and dry at 80°C for 12 hours to obtain Al(OH) 3 solid. Measure a certain amount of Mn (NO 3 ) 2 solution and Ce(NO 3 ) 2 solid, add a small amount of deionized water to make Ce(NO 3 ) 2 The solid dissolves and the resulting Al(OH) 3 The solid was impregnated with Mn(NO 3 ) 2 and Ce(NO 3 ) 2 in the mixed solution for 12h, and then the impregnated product was dried in a water bath at 80°C for 12h, and then placed in an oven at 110°C for 2h to obtain a black solid. Then put the black solid in a muffle furnace for calcination at 500°C for 6 hours, grind it, and pass through a 40-60 mesh sieve to obtain the catalyst Mn-Ce(0.20) / γ-Al 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com