Method for extracting indole from methyl naphthalene fraction
A technology of methylnaphthalene fraction and indole, which is applied in the field of indole extraction, can solve the problems of low quality and low yield of indole oil, and achieve the effects of high yield, short process and less investment in equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
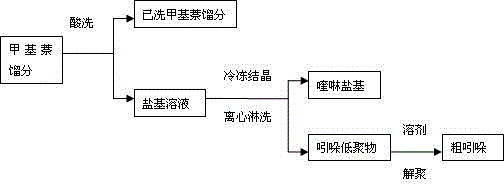
[0029] Add the methylnaphthalene fraction containing 3% indole to the scrubber, add sulfuric acid solution with a concentration of 50% according to the ratio of mass ratio 1:0.1, heat up to 60°C under stirring conditions, keep it warm for 90min, and let it stand for 60min before releasing The quinoline base solution of indole oligomers is dissolved in the lower layer, and finally the washed methylnaphthalene fraction is released, which basically does not contain quinoline homologues and indole. The solution in the lower layer was frozen to -5°C, and then centrifuged to obtain khaki granular indole oligomer salt, poured into 5% sodium hydroxide solution under the condition of centrifuge rotation until the centrifuge was alkaline, and then added the solid material In the reaction kettle with a reflux condenser, add the solvent crude dimethylethylbenzene in a mass ratio of 1:0.5, raise the temperature to 140°C under stirring conditions, keep the temperature for 90min, and then gra...
Embodiment 2
[0031] Add the methylnaphthalene fraction containing 4% indole to the scrubber, add the sulfuric acid solution with a concentration of 55% according to the mass ratio of 1:0.15, raise the temperature to 70°C under stirring, keep it warm for 90min, and let it stand for 60min, then release A quinoline base solution of indole oligomers is dissolved in the lower layer, and then the washed methylnaphthalene fraction is released, which basically does not contain quinoline homologues and indole. The solution in the lower layer was frozen to -3°C, and then centrifuged to obtain khaki granular indole oligomer salt, poured into 5% sodium hydroxide solution under the condition of centrifuge rotation until the centrifuge was alkaline, and then added the solid material In the reaction kettle with a reflux condenser, add the crude dimethylethylbenzene as a solvent in a mass ratio of 1:0.5, heat up to 150°C under stirring conditions, keep the temperature for 90min, and then gradually increase...
Embodiment 3
[0033]Add the methyl naphthalene fraction containing 5% indole to the scrubber, add the sulfuric acid solution with a concentration of 60% according to the mass ratio of 1:0.2, raise the temperature to 80°C under stirring, keep it warm for 90min, and let it stand for 60min, then release The quinoline base solution of indole oligomers is dissolved in the lower layer, and finally the washed methylnaphthalene fraction is released, which basically does not contain quinoline homologues and indole. The solution in the lower layer was frozen to -2°C, and then centrifuged to obtain khaki granular indole oligomer salt, poured into 5% sodium hydroxide solution under the condition of centrifuge rotation until the centrifuge was alkaline, and then added the solid material In the reaction kettle with a reflux condenser, add the crude dimethylethylbenzene as a solvent in a mass ratio of 1:0.5, heat up to 150°C under agitation, keep the temperature for 90min, and then gradually increase the v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com