Crystallization form of dihydromyricetin, preparation method thereof and pharmaceutical composition containing the same
A technology of dihydromyricetin and crystal form, applied in the field of medicine, can solve the problems of poor solubility, unfavorable preparation of pharmaceutical raw materials, storage, influence on quality stability, etc., and achieves the effects of simple preparation method, stable crystal form and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Take 1g of crude dihydromyricetin, add 2ml of acetone to dissolve, then add 2ml of glacial acetic acid to the solution, shake well, and place it at room temperature for crystallization. The obtained crystals were filtered, and after standing at room temperature for 24 hours, they were placed in an oven and dried at 100°C for 24 hours to obtain Form I.
Embodiment 2
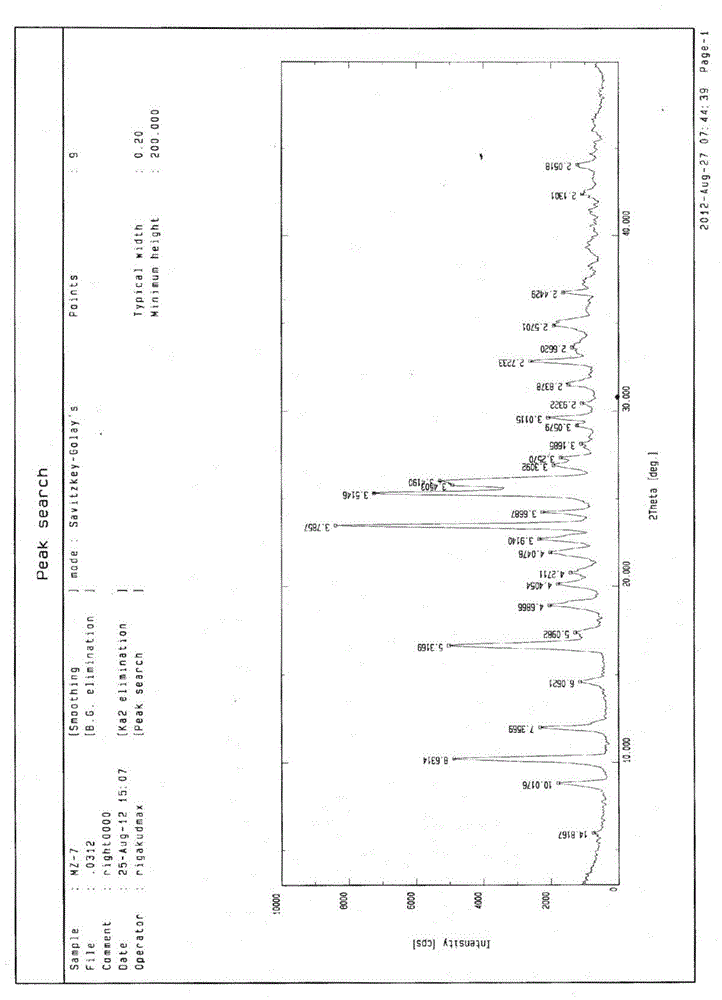
[0027] Take 1g of crude dihydromyricetin, add 3ml of acetone to dissolve, then add 6ml of glacial acetic acid to the solution, shake well, and place it at room temperature for crystallization. The obtained crystals were filtered, placed at room temperature for 24 hours, then placed in an oven, and dried at 105°C for 24 hours to obtain Form I. With Cu-Ka radiation, the X-ray powder diffraction pattern of the crystalline form I of dihydromyricetin represented by the 2θ angle and the interplanar spacing is shown in the appendix figure 1 , see Table 1 for the description of the spectrum.
[0028] Table 1
[0029]
Embodiment 3
[0031] Take 1g of crude dihydromyricetin, add 2ml of acetone to dissolve, then add 8ml of glacial acetic acid to the solution, shake well, and place it at room temperature for crystallization. The obtained crystals were filtered, left at room temperature for 12 hours, placed in an oven, dried at 80°C for 24 hours, and then dried at 120°C for 12 hours to obtain Form I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com