siRNA composition for treating viral hepatitis B
A viral hepatitis and composition technology, applied in the field of medicine and biology, can solve the problems of packaging, poor protection effect, insufficient drug effect, large drug dosage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
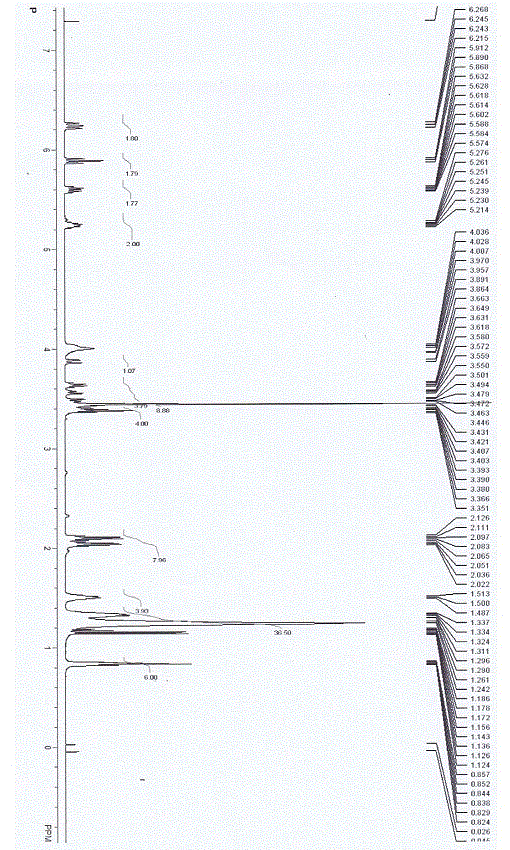
[0054] Put epoxyalkyl glycidyl ether (alkyl C12) and 1,4,7,10-tetraazacyclododecane in a molar ratio of 1:4 into a glass bottle with a stirring bar, and react at 90°C for 36 Hour. The reaction products were subjected to TLC analysis, and there was only one main product, which was purified and used to prepare the interfering composition.
[0055] (1) Dissolve 6.25 parts of ID No.267 in water or water containing 9% sucrose to obtain 1L of solution;
[0056] (2) Weigh 1 part of trimethyl [2,3-(dilinoleyloxy) propyl] ammonium chloride, 0.5 part of cylindrine, 1 part of phosphatidylethanolamine, 5 parts of T2C1 compound, cholesterol 1 part, 1 part of cholesterol polyethylene glycol and 3 parts of dimyristoyl glycerol polyethylene glycol, and dissolve these components in alcohol to obtain 0.25 L of solution 2;
[0057] (3) After the solution 1 and the solution 2 are mixed uniformly, vacuum filtration is carried out at room temperature, so that the alcohol therein is gradually evap...
Embodiment 2
[0061] (1) Dissolving 0.2 parts of the siRNA sequence in water or water containing 9% sucrose to obtain a solution of 1 L;
[0062] (2) Weigh 4 parts of T2C1 compound and dissolve it in alcohol to obtain 0.25L of solution 2;
[0063] (3) After the solution 1 and the solution 2 are mixed uniformly, vacuum filtration is carried out at room temperature, so that the alcohol therein is gradually evaporated;
[0064] (4) After most of the alcohol evaporates, homogeneously obtain the particles of the suspension to be 60nm;
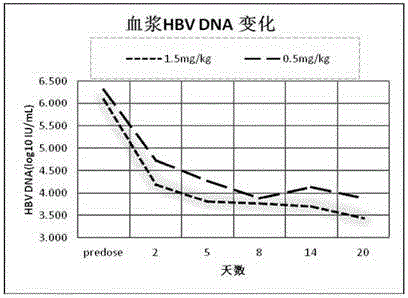
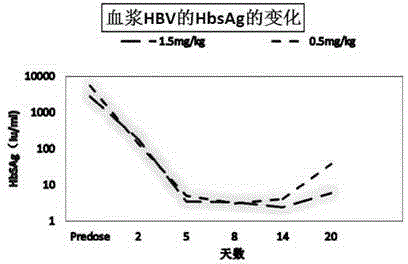
[0065] (5) Perform high-pressure homogenization and freeze-drying on the suspension to form a dried product, and obtain the RNA interference composition for treating viral hepatitis B.
Embodiment 3
[0067] (1) Dissolving 0.5 parts of the siRNA sequence in water or water containing 9% sucrose to obtain a solution of 1 L;
[0068] (2) Weigh 6 parts of T2C1 compound and dissolve it in alcohol to obtain 0.5L of solution 2;
[0069] (3) After the solution 1 and the solution 2 are mixed uniformly, vacuum filtration is carried out at room temperature, so that the alcohol therein is gradually evaporated;
[0070] (4) After most of the alcohol is evaporated, homogenize, and the particles of the suspension are 120nm;
[0071] (5) Perform high-pressure homogenization and freeze-drying on the suspension to form a dried product, and obtain the RNA interference composition for treating viral hepatitis B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com