Hog cholera virus genetic recombination adenovirus carrier vaccine rabbit body effect detection method
A technology of gene recombination and swine fever virus, applied in the field of biotechnology detection, can solve the problems of no stable, simple and cost-saving vaccine efficacy evaluation standards, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Establishment of the Efficacy Testing Method for Rabbit Body of New Type CSFV Gene Recombinant Adenovirus Vector Vaccine
[0018] 1.1 Experimental poisons, vaccines, and experimental animals
[0019] Recombinant adenovirus vaccine for classical swine fever (rAd-E0-E2, ≥2×10 7.0 IFU / bottle, the determination method is detailed in the patent: Zhang Heng, Fan Gencheng, Du Yuanzhao, etc. A method for determining the virus content of the recombinant adenovirus vector vaccine of classical swine fever virus [P]. The State Intellectual Property Office of the People's Republic of China. CN 104535764 A, 2015.04.22), was freeze-dried and preserved by the technology center of Qingdao Yibang Bioengineering Co., Ltd.; live swine fever vaccine (rabbit source) referred to as swine fever spleen leaching virus, batch number 201401, 20 heads / bottle, produced by the freeze-dried seedling workshop of the company ; 1.5-3kg healthy susceptible rabbits were purchased from Qingdao K...
Embodiment 2
[0035] Embodiment 2: Rabbit body potency testing method determines the minimum immune dose of rAd-E0-E2
[0036] The minimum immune dose of rAd-E0-E2 is determined according to the rabbit body efficacy test method established in Example 1, and the steps are as follows:
[0037] 2.1 Immunization method Carry out immunization according to the method in Table 1 in step 1.2.
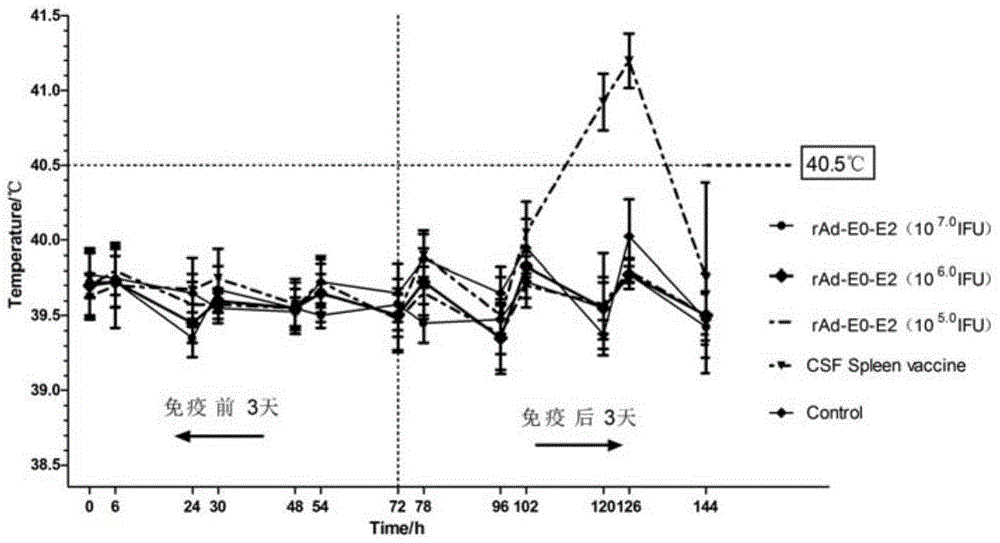
[0038] 2.2 Immunization methods and actual temperature measurement results before and after immunization The body temperature was measured in the morning and afternoon for 3 consecutive days before and after immunization.
[0039] 2.3 Protective effect of rAd-E0-E2 on immunized rabbits
[0040] After attacking the poison, the original multiplier (10 7.0 IFU) immunized rabbits and CSF splenic gonorrhea immunized rabbits had body temperature 6.0 3 / 4 of IFU) immunized rabbits had a stereotyped thermal reaction, 3 / 4 of the rabbit spleen / body mass index ratio was relatively large, 3 / 4 of the spleen tissue secti...
Embodiment 3
[0045] Embodiment 3: Rabbit physical effect test method evaluates the effectiveness of 3 batches of freeze-dried rAd-E0-E2 vaccines
[0046] Get 3 batches of freeze-dried rAd-E0-E2 vaccine (potency ≥ 2 × 10 7.0 IFU / bottle), named as freeze-dried vaccine 1, freeze-dried vaccine 2 and freeze-dried vaccine 3 respectively. The 3 batches of vaccines were immunized according to the method in Table 1 in 1.2, and the five evaluation methods of the effect test model established in the specific example 1 were used for comparison and detection. The results are shown in Tables 3-5. According to the comparative test results, it was proved that the potency of the 3 batches of freeze-dried vaccines was relatively stable, and it was further determined that the minimum immune dose of the vaccines was all at 10 7.0 IFU protection, and further prove the stability of the rabbit physical examination method.
[0047] Table 3 Comparison of protection results of five evaluation methods after immuni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com