Open tubular capillary column with nano-crystalline cellulose derivative modified surface and application of open tubular capillary column with nano-crystalline cellulose derivative modified surface
An open-tube capillary column and nanocellulose technology, which is applied in other chemical processes, solid adsorbent liquid separation, instruments, etc., can solve the problems of chiral separation of nanocellulose, achieve satisfactory separation effect, and simple preparation method , low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
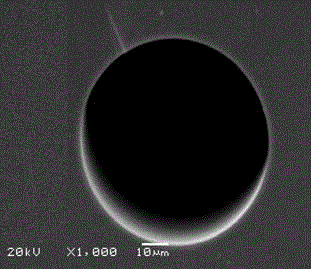
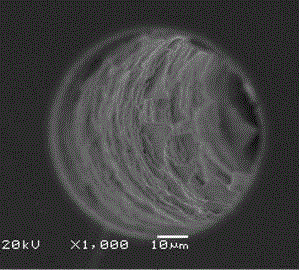
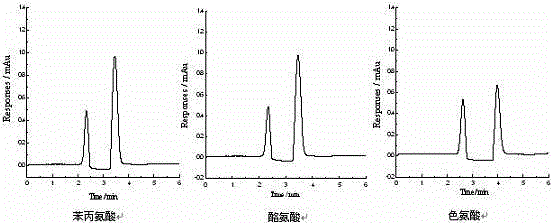
Image
Examples
Embodiment 1
[0022] 10 g of microcrystalline cellulose was hydrolyzed with 64% concentrated sulfuric acid, and after several times of centrifugation, the unhydrolyzed cellulose was removed to obtain nanocellulose, which was about 3 g after freeze-drying; 3 g of nanocellulose was protected by 6-position Methods Modification of 3,5-dimethylphenylisocyanate on nanocellulose. Activate the capillary with 0.1 mol / L NaOH, distilled water, 0.1 mol / L HCl, and distilled water in sequence. Then fix the treated capillary on the pump head, and at a flow rate of 0.1mL / min, first pass 10 mol / L cetyltrimethylammonium bromide into the capillary, and adsorb on the inner wall of the capillary by electrostatic action, Then use N 2 Purge until no solution flows out; then dissolve the above-prepared nanocellulose derivatives in pyridine and add them to the ethanol solution of ethyl orthosilicate, the concentration of nanocellulose derivatives is 0.1 mg / mL, and stir under ice bath conditions After 4 hours of r...
Embodiment 2
[0024] 10 g of microcrystalline cellulose was hydrolyzed with 64% concentrated sulfuric acid, and after several times of centrifugation, the unhydrolyzed cellulose was removed to obtain nanocellulose, which was about 3 g after freeze-drying; 3 g of nanocellulose was protected by 6-position Methods Modification of 3,5-dimethylphenylisocyanate on nanocellulose. Activate the capillary with 0.1 mol / L NaOH, distilled water, 0.1 mol / L HCl, and distilled water in sequence. Then fix the treated capillary on the pump head, and at a flow rate of 0.2 mL / min, first pass 20 mol / L cetyltrimethylammonium bromide into the capillary, and adsorb on the inner wall of the capillary by electrostatic action, Then use N 2 Purge until no solution flows out; then dissolve the above-prepared nanocellulose derivatives in pyridine and add them to the ethanol solution of ethyl orthosilicate, the concentration of nanocellulose derivatives is 0.2 mg / mL, and stir under ice bath conditions After 4 hours of ...
Embodiment 3
[0026] 10 g of microcrystalline cellulose was hydrolyzed with 64% concentrated sulfuric acid, and after several times of centrifugation, the unhydrolyzed cellulose was removed to obtain nanocellulose, which was about 3 g after freeze-drying; 3 g of nanocellulose was protected by 6-position Methods Modification of 3,5-dimethylphenylisocyanate on nanocellulose. Activate the capillary with 0.1 mol / L NaOH, distilled water, 0.1 mol / L HCl, and distilled water in sequence. Then fix the treated capillary on the pump head, and at a flow rate of 0.3 mL / min, first pass 40 mol / L cetyltrimethylammonium bromide into the capillary, and adsorb on the inner wall of the capillary by electrostatic action, Then use N 2 Purge until no solution flows out; then dissolve the above-prepared nanocellulose derivatives in pyridine and add them to the ethanol solution of ethyl orthosilicate, the concentration of nanocellulose derivatives is 0.4 mg / mL, and stir under ice bath conditions After 4 hours of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com