Construction method of whole-genome exon sequence capture probe
A sequence capture and genome-wide technology, applied in recombinant DNA technology, DNA preparation, DNA/RNA fragments, etc., can solve the problems of limited species capture and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
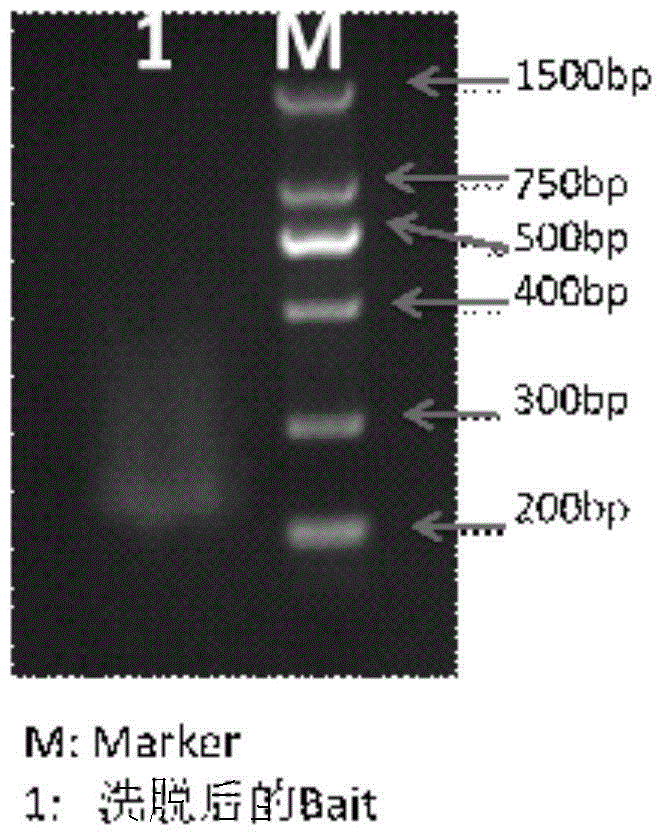
Image
Examples
Embodiment 1
[0055] 1. Extraction of total RNA from normal human blood
[0056] Subjects: normal people
[0057] Sample collection method: blood collection with anticoagulant tubes, and immediately put the blood collection tubes into the ice box
[0058] Experimental steps:
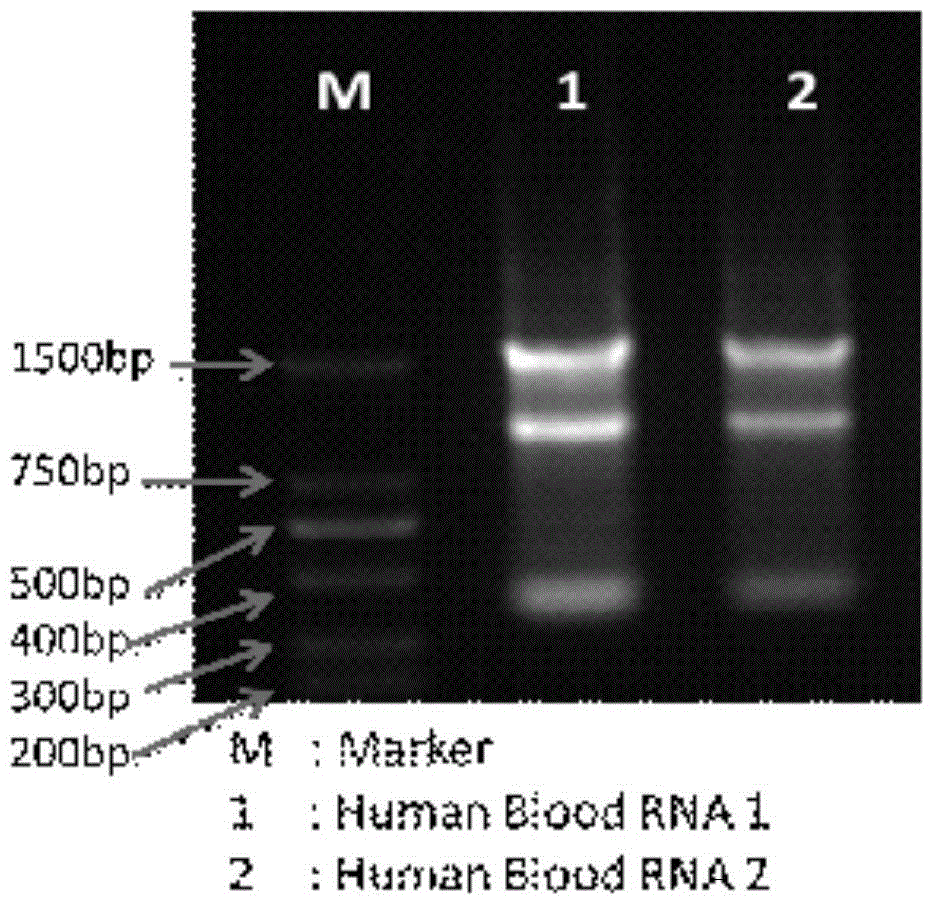
[0059] Ribonucleic acid quality inspection: use Trizol method to extract normal human blood, Trizol method for mRNA extraction is relatively mature, you can refer to textbooks such as Molecular Cloning Experiment Guide, or use existing commercially available kits, such as invitrogen; No.15596- 026 kit, perform gel electrophoresis detection on the extracted RNA (see Chapter 5 of the third edition of Molecular Cloning), and see the results in figure 1 .
[0060] 2. Capturing exon sequences
[0061] (1) Interrupting mRNA
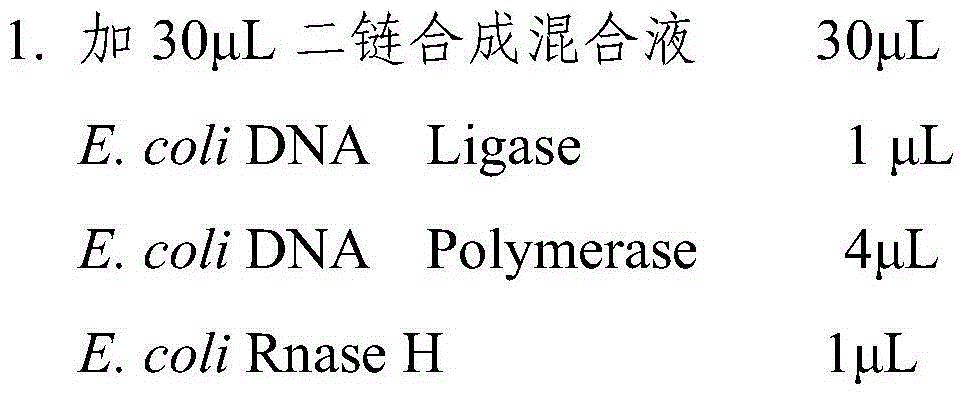
[0062] Dilute 4ug of total RNA with 50 μL of nuclease-free ultrapure water, add purified magnetic beads (RNA Purification Beads 15026778illumina) at a volume ratio of 1:1, incubate at 65°C for 5 m...
Embodiment 2
[0121] Use the peripheral blood of patients with depression, use Trizol to extract blood RNA, and then conduct experiments according to the technical scheme of Example 1, construct exon capture reagents, and conduct experiments. The results are analyzed by bioinformatics, after removing joints and removing low-quality After the data filtering operation, high-quality sequences were obtained, compared with the reference genome through bwa and converted and calibrated using picard and GATK software, and statistics were performed, and it was known that the effective data we measured could cover more than 90% of the exons
[0122] More than 90% of exons were successfully covered.
[0123]
Embodiment 3
[0125] Using the peripheral blood of rats, Trizol was used to extract blood RNA, and then the above scheme was used to conduct experiments, to construct exon capture reagents, and to conduct experiments. The results were analyzed by bioinformatics, and after data filtering operations such as removing connectors and removing low-quality data , to obtain a high-quality sequence, compare it with the reference genome through bwa and use picard, GATK software to convert and calibrate, and then perform statistics. It is known that the effective data we measured can cover more than 90% of the exons.
[0126]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com