Preparation method of 17,21-dihydroxy steroid derivative
A technology of bishydroxysteroid and steroid enol silyl ether, which is applied in the field of preparation of 17,21-bishydroxysteroid derivatives, can solve the problems of no literature report and the like, achieves simple operation, high yield and easy industrialization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
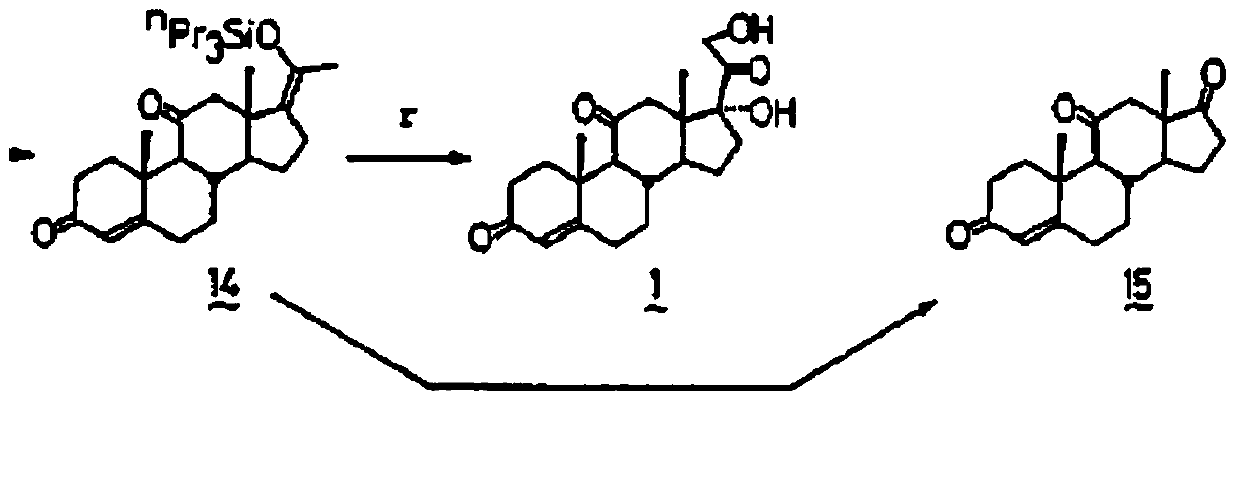
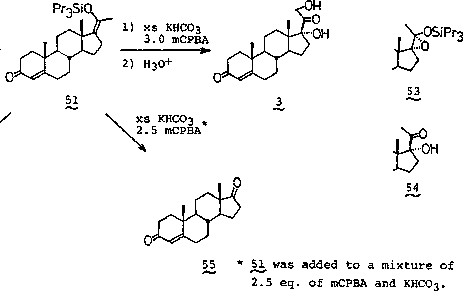
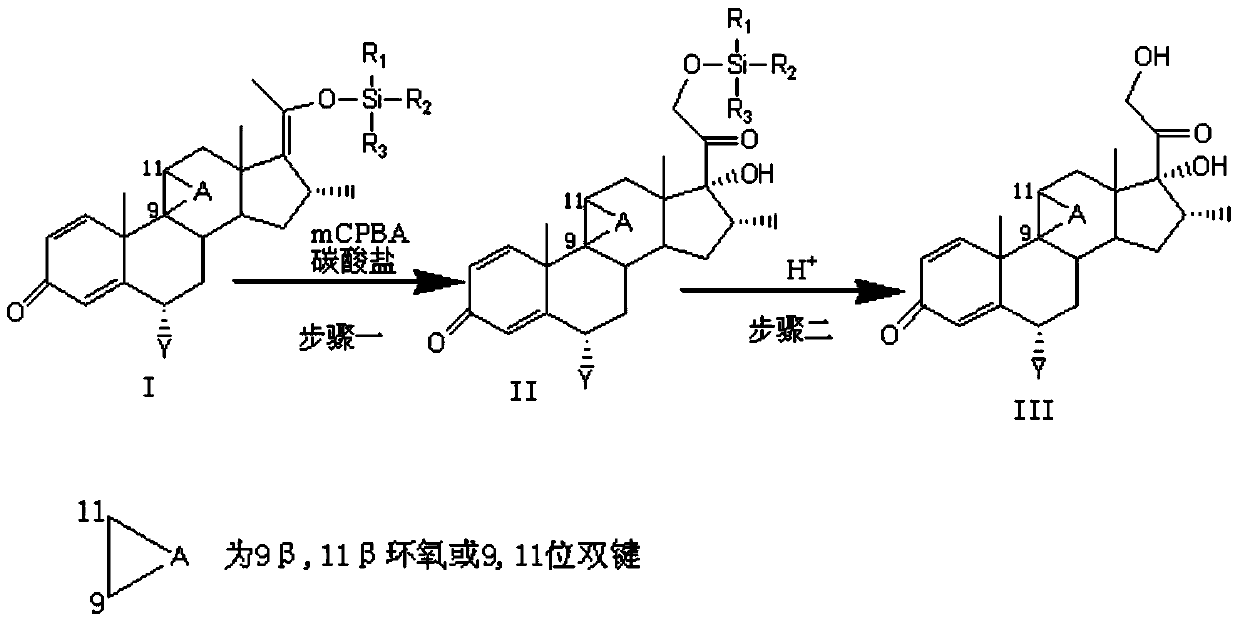
[0049] Example 1 Preparation of 17,21-dihydroxy compound AIII with enol silicon ether compound AI as starting material
Embodiment 1-1
[0051] Take compound AI (100g, 0.23mol) and dissolve it in 1L dichloromethane solution; take mCPBA (85%, 162.4g, 0.8mol) and dissolve it in 3.25L dichloromethane solution and add powdery dry KHCO 3 (70g, 0.7mol); combine mCPBA and KHCO 3 The mixed solution was added to the dichloromethane solution of compound AI cooled to 0°C in 1 hour. The temperature of the reaction system was controlled to 0~5°C, and the reaction system was stirred while adding. Thin layer chromatography was used to monitor the progress of the reaction. After the reaction is complete, add an appropriate amount of 10% sodium thiosulfate aqueous solution to remove the excess mCPBA, use starch potassium iodide test paper to test the non-oxidation of the reaction solution, add 1L water, separate the layers, separate the organic layer, and use 1L dichloromethane for the water layer Extract once, combine the organic layers and wash with water to neutrality, concentrate and nearly dry to obtain crude AII. The crude A...
Embodiment 1-2
[0053] Take compound AI (100g, 0.23mol) and dissolve it in 1L dichloromethane solution; take mCPBA (85%, 162.4g, 0.8mol) and dissolve it in 3.25L dichloromethane solution and add powdered dry KHCO3 (70g, 0.7mol) ); put mCPBA and KHCO 3 The mixed solution was added to the dichloromethane solution of compound AI cooled to 10°C within 1 hour, and the temperature of the reaction system was controlled to 10°C±2°C. Stirring while adding, the progress of the reaction was monitored by thin layer chromatography After the reaction is complete, add an appropriate amount of 10% sodium thiosulfate aqueous solution to remove excess mCPBA, use starch potassium iodide test paper to test the non-oxidation of the reaction solution, add 1L of water, separate the layers, separate the organic layer, and use 1L of dichloride for the water layer. Methane was extracted once, the combined organic layer was washed with water to neutrality, concentrated to near dryness to obtain crude AII. The crude AII o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com