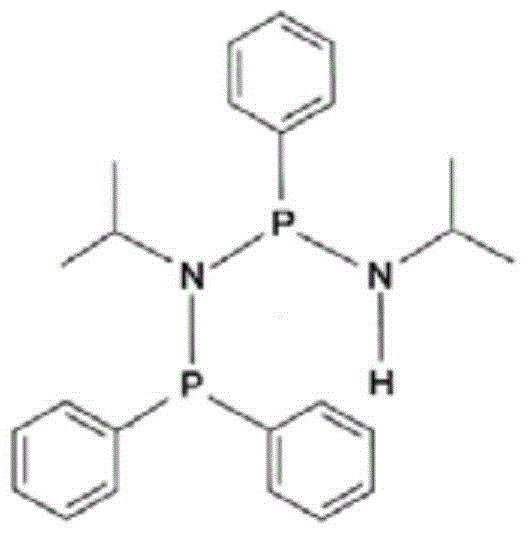
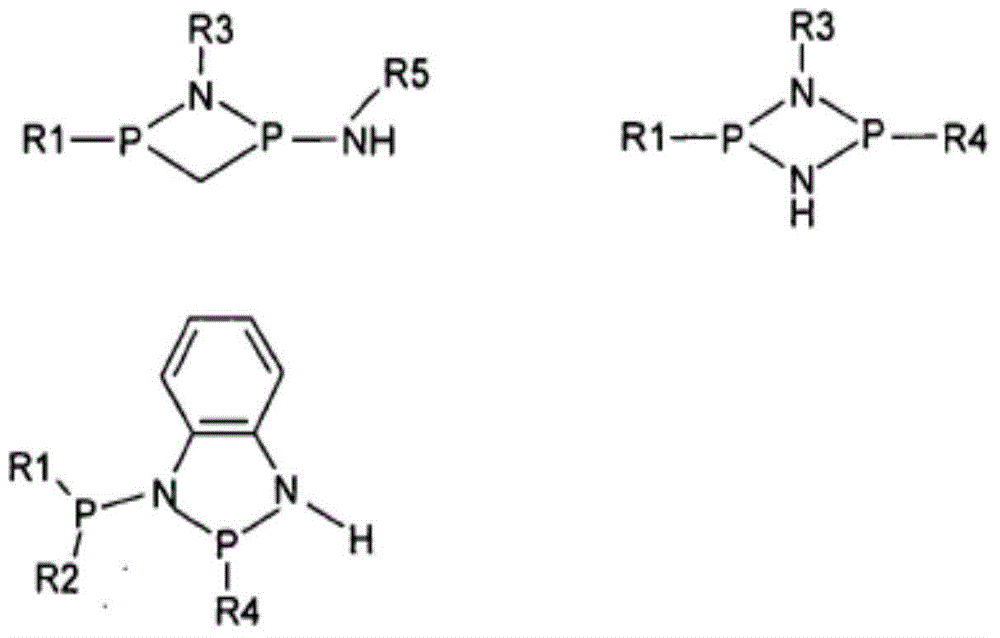
Method for purifying a crude pnpnh compound
A compound, R1R2P-N technology, applied in the field of purifying crude PNPNH compounds, can solve problems such as unsuccessful
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Ligand Preparation, Laboratory Scale
[0054] Preparation of Bis(isopropyl-amino-)phenylphosphine(NPN)
[0055] To a stirred solution of isopropylamine (30ml, 352mmol) in ether (250ml) was added dichlorophenylphosphine (9.63ml, 71mmol, dissolved in 50ml ether) at 0°C over a period of 30min. After stirring for a total of 72 h, the solution was filtered. The residue was washed with ether and the solvent was removed in vacuo. The remaining oil was distilled at 0.2 Torr / 76-78 °C to give a colorless liquid in 33% yield (5.3 g). 31 P{H} NMR: 49.0 ppm.
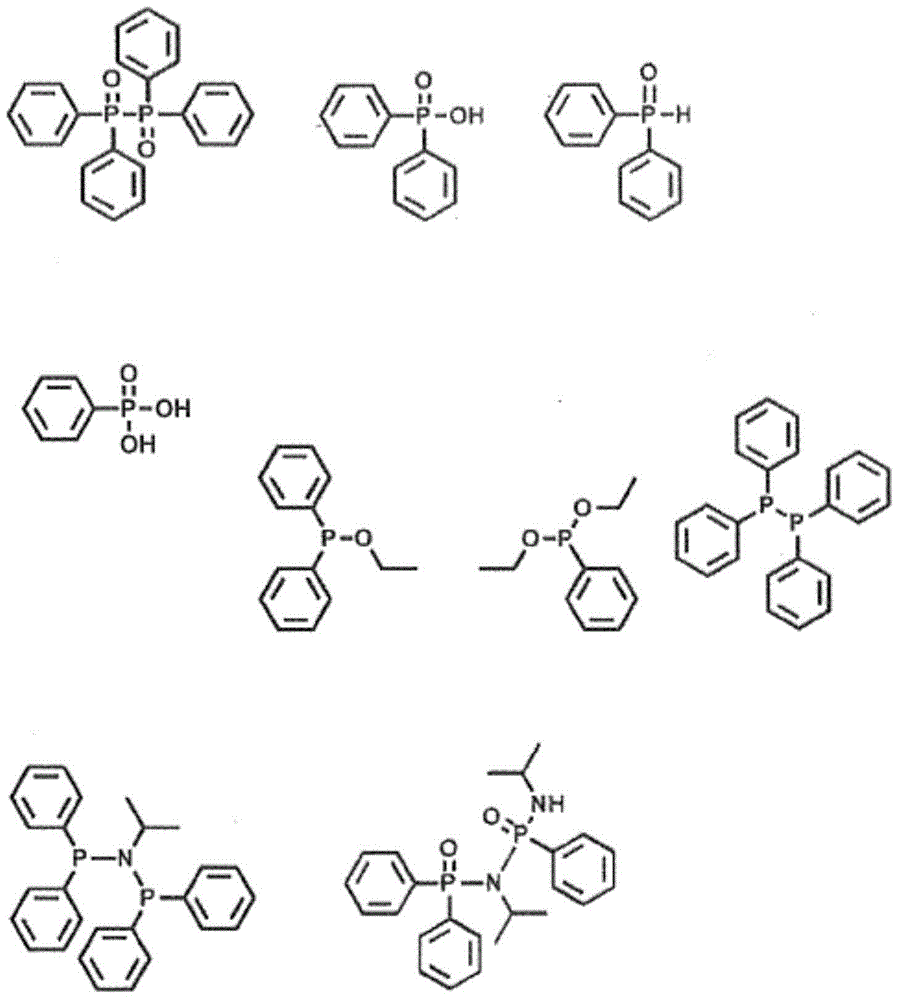
[0056] After synthesis at crude Ph 2 Some of the impurities found in P-N(i-Pr)-P(Ph)-N(i-Pr)-H ligands are shown below.
[0057]
[0058] (Ph) 2 Preparation of PN(i-Pr)P(Ph)NH(i-Pr)(PNPN-H)
[0059] A solution of NPN species (2.4 g, 10.7 mmol) in tetrahydrofuran (10 ml) was added dropwise to triethylamine (6 ml) and chlorodiphenylphosphine (2.36 g, 10.7 mmol) in THF (40 ml) at −40° C. ) in a stirred solut...
Embodiment 2
[0061] [Ph 2 PN(i-Pr)P(Ph)N(i-Pr)-Li] 2 preparation of
[0062] Ph 2 PN(i-Pr)P(Ph)N(i-Pr)-H (8.70 g, 21.35 mmol) was dissolved in 15 ml of toluene. After cooling to -78°C, n-butyllithium (12.8 ml, 2.5M n-BuLi in n-heptane, 32.0 mmol) was added to the solution, causing an immediate orange / yellow color change. The solution was stirred at room temperature for an additional two hours and a colorless solid precipitated. The precipitate was filtered and washed 3 times with 5 ml toluene. The remaining solvent was removed under vacuum to yield a colorless powder. Yield: 6.73 g (76%). Molecular weight: 414.39g / mol[C 24 h 29 LiN 2 P 2 ]. Elemental Analysis: Calculated: C 69.56%, H 7.05&, N 6.76%; Found: C 69.25%, H 7.06%, N 6.87%. Melting point: 187-189°C. 1 H NMR (THF-d 8 ) δ = 7.50-7.57 (m, 6H, aryl-H), 7.20-7.34 (m, 6H, aryl-H), 7.02 (m, 2H, aromatic), 6.93 (m, 1H, aryl- H), 3,70(m, 1H, CHCH 3 ), 3.58 (m, 1H, CHCH 3 ), 1.39 (d, J=6.47Hz, 3H, CHCH 3 ), 1.25 (d, J=6.2...
Embodiment 3
[0063] Example 3: Ligand purification via metallation of PNPN-H ligand with subsequent reprotonation
[0064]The crude ligand (8.70 g, 21.35 mmol) was dissolved in 15 ml of toluene. n-BuLi (12.8 ml, 2.5M n-BuLi in n-heptane, 32.0 mmol) was added to the solution with cooling, causing an immediate orange / yellow color change. The solution was stirred at room temperature for an additional two hours and a colorless solid precipitated. The precipitate was filtered and washed with 5 ml of toluene. The residue and ammonium chloride (1.70 g, 32 mmol) were suspended in toluene. The suspension was stirred for 10 h. Filtration yielded a toluene solution of purified ligand which was used in the catalytic reaction without further workup. Alternatively, the solvent was removed in vacuo to yield 5.41 g (62%). Molecular weight: 408.19g / mol[C 24 h 30 N 2 P 2 ]. Elemental Analysis: Calculated: C 70.57%, H 7.40%, N 6.86%; Found: C 70.50%, H 7.35%, N 6.87%.
[0065] Furthermore, the lit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com