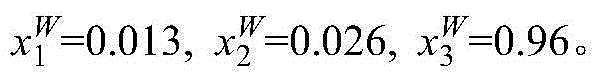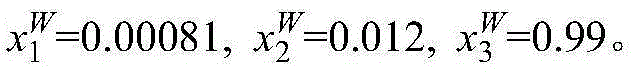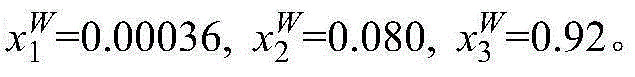Extraction separation method for methacrylic acid by using ionic liquid
A methacrylic acid, ionic liquid technology, applied in the separation/purification of carboxylic acid compounds, organic chemistry and other directions, can solve the problems of low extraction capacity of methacrylic acid, environmental and safety problems, a large number of extractants, etc., to achieve strong extraction capacity , hazard reduction, high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0007] Add 10 g of 1% methacrylic acid aqueous solution and 5 g of [C 6 MIM](CF 3 SO 2 ) 2 The N ionic liquid was heated by a circulating water bath, and the temperature was controlled at 298.15K. The glass kettle was placed on a magnetic stirrer, and after magnetic stirring for 20mim, the magnetic stirrer was turned off and left to stand. The system is divided into two layers, the upper layer is the water phase, and the lower layer is the ionic liquid phase. The water phase and the ionic liquid phase are sampled respectively, and the contents of methacrylic acid and ionic liquid in the sampling are analyzed by ultra-high liquid chromatography. 1 and x 2 , and then calculate the water content x in the two phases by the difference method 3 . The specific measurement results are: in the lower ionic liquid phase, in the upper aqueous phase x 2 W = 0 , x 3 ...
Embodiment 2
[0014] Take 2g mass fraction of 40% methacrylic acid aqueous solution and 1g [CH 3 -N-(C 8 h 17 ) 3 ]Cl ionic liquid is placed in a jacketed glass kettle, heated by a circulating water bath, and the temperature is controlled at 298.15K. The glass kettle is placed on a magnetic stirrer and stirred and mixed for 20mim, and then the magnetic stirrer is turned off to stand for stratification. The upper layer is ionic liquid phase, the lower layer is the water phase. The two phases are sampled respectively, and the content of methacrylic acid and ionic liquid in the sample is analyzed by ultra-high liquid chromatography to be x respectively 1 and x 2 , and then calculate the water content x in the two phases by the difference method 3 . The specific measurement results are: in the upper ionic liquid phase, x 1 I L = 0.41 , x 2 ...
Embodiment 3
[0016] Take 10g mass fraction of 1% methacrylic acid aqueous solution and 5g of [CH 3 -N-(C 8 h 17 ) 3 ]Cl ionic liquid is placed in a jacketed glass kettle, heated by a circulating water bath, and the temperature is controlled at 298.15K. The glass kettle is placed on a magnetic stirrer and stirred and mixed for 20mim, and then the magnetic stirrer is turned off to stand for stratification. The upper layer is ionic liquid phase, the lower layer is the water phase. The two phases are sampled respectively, and the content of methacrylic acid and ionic liquid in the sample is analyzed by ultra-high liquid chromatography to be x respectively 1 and x 2 , and then calculate the water content x in the two phases by the difference method 3 . The specific measurement results are: in the upper ionic liquid phase, x 1 I L = 0.022 , x 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com