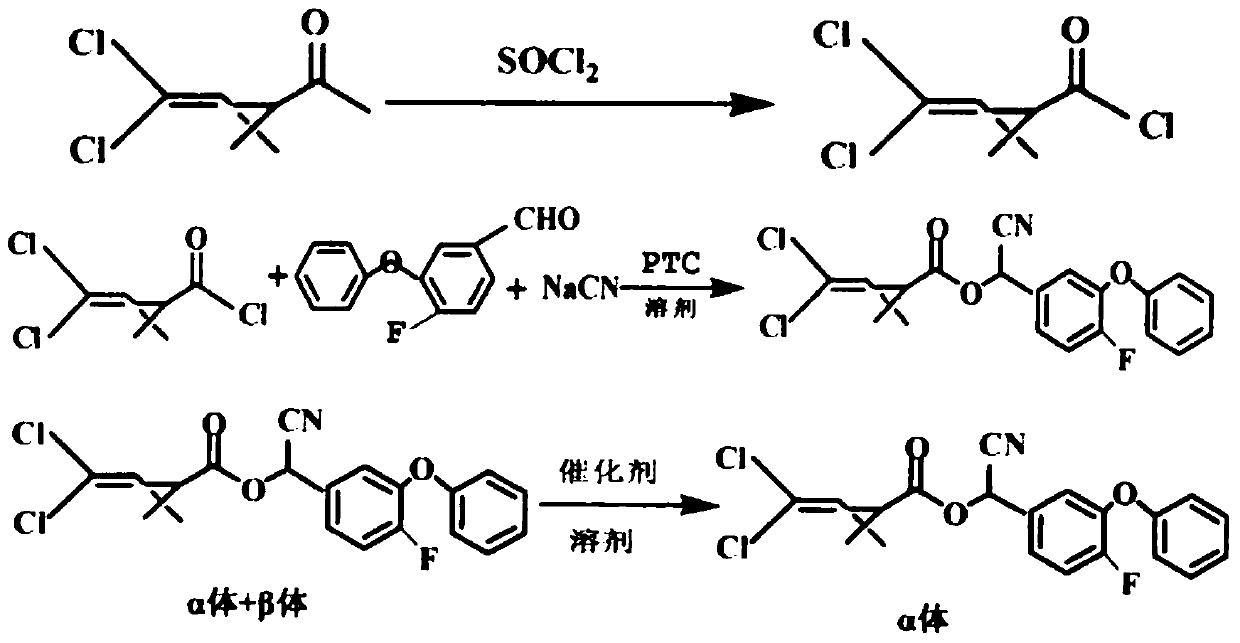
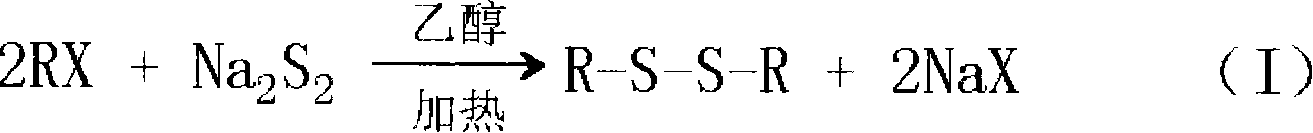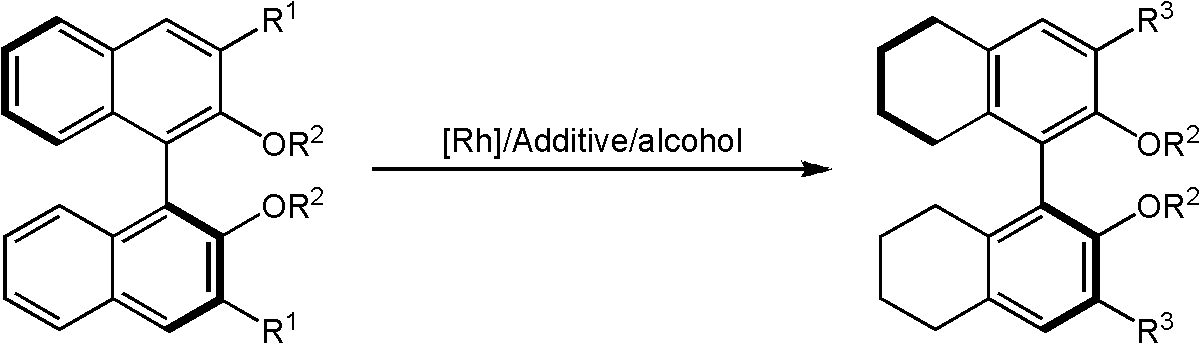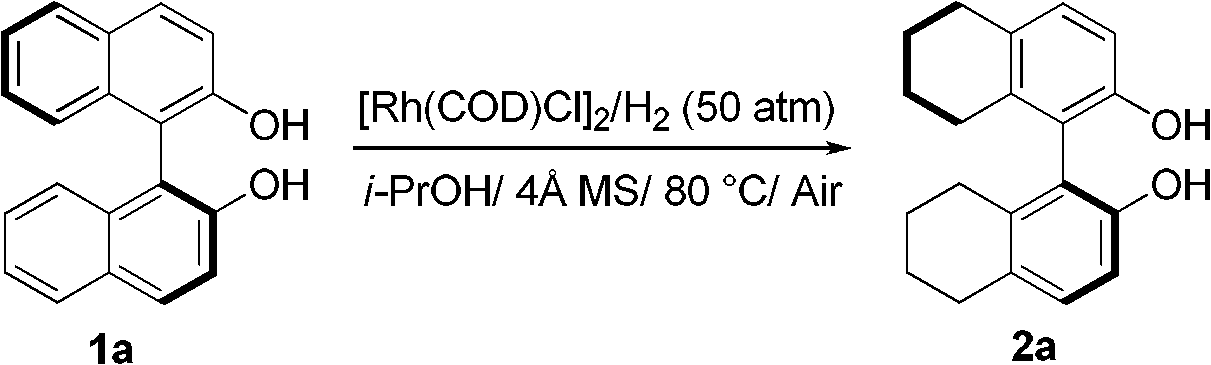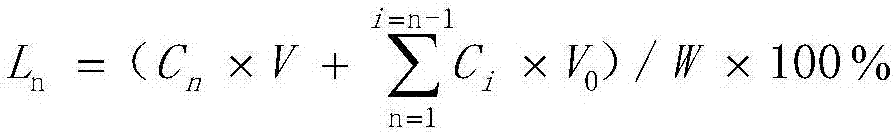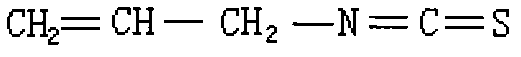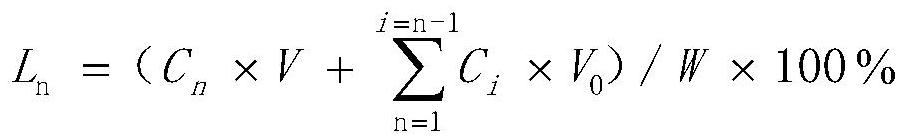Patents
Literature
53 results about "Trioctylmethylammonium chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
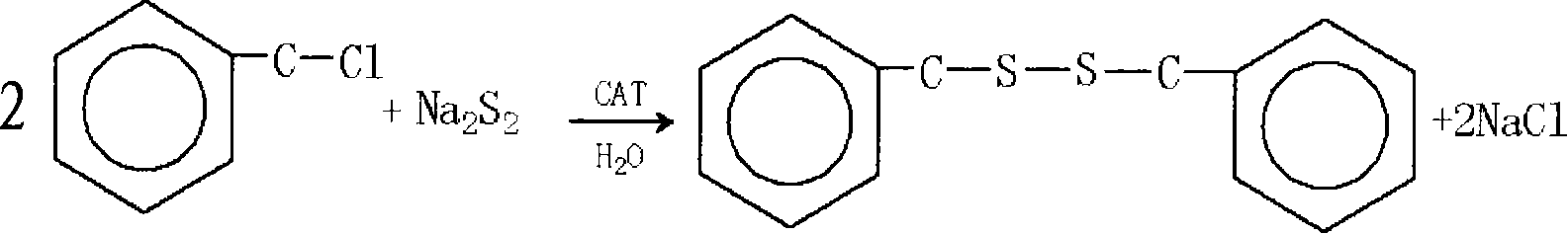
Method for preparing dibenzyl disulfide
InactiveCN101607930AHigh yieldDelayed reaction timeHydropoly/poly sulfide preparationReaction temperatureBenzyl chloride
The invention relates to a method for preparing dibenzyl disulfide. The innovation points of the invention are that: water is taken as a reaction solvent; Na2S2, benzyl chloride and a catalyst are mixed and then react; the reaction temperature is between 30 and 50 DEG; the reaction time is between 10 and 40 minutes; the temperature of a reaction liquid is cooled to 20 DEG C after the reaction is finished; the pH value is adjusted to 7; and a separated organic phase is washed, dried and purified to obtain the dibenzyl disulfide. The method uses trioctylmethylammonium chloride as the catalyst to perform a catalytic reaction on the mixed Na2S2 and benzyl chloride, and has high yield of finished products which is improved by 15 to 20 percent compared with the prior synthetic method; and the method greatly reduces reaction time and reaction temperature, is easy to control reaction process, has few byproducts, and reduces the consumption of energy sources.
Owner:TIANJIN CHEM REAGENT RES INST
Preparation method of an iron and carbohydrate complex
InactiveCN108129582AStable molecular weightSmall polydispersity indexHypochloriteQuaternary ammonium cation
The invention provides a preparation method of an iron and carbohydrate complex, and particularly relates to a preparation method of ferric carboxymaltose. The preparation method of ferric carboxymaltose comprises the following steps: maltodextrin is oxidized by an oxidant containing tert-butyl hypochlorite in an alkaline aqueous solution in the presence of a phase transfer catalyst; the oxidizedmaltodextrin solution is subjected to a reaction with an aqueous solution of ferric iron salt, wherein the phase transfer catalyst is selected from one or more of quaternary ammonium salts such as trioctylmethylammonium chloride, tributylmethylammonium chloride, tributylmethylammonium bromide, tributylmethylammonium chloride, tetrabutylammonium chloride, and tetrabutylammonium bromide; the oxidantis tert-butyl hypochlorite or a combination of tert-butyl hypochlorite and sodium hypochlorite. Obtained ferric carboxymaltose has MW (molecular weight) of 150-230 kDa, Pd value of 1.2-1.3 and iron content of 26%-31%.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Novel process for preparing benzyl alcohol by alkali-free continuous hydrolysis of benzyl chloride
ActiveCN104926611ATransfer out in timeEasy to handleOrganic compound preparationHydroxy compound preparationAlkali freeDistillation
The invention relates to a novel process for preparing benzyl alcohol by alkali-free continuous hydrolysis of benzyl chloride. The process comprises the following steps of adding benzyl chloride, pure water and a specific mixed solvent of methylbenzene and cyclohexane into a primary normal-pressure reaction kettle according to a certain proportion, performing reaction for a certain time at certain temperature, continuing transferring a reaction product into a secondary pressure reaction kettle, adding an isopropylbenzene solvent, simultaneously adding methyl trioctyl ammonium chloride quaternary ammonium salt, continuing performing reaction for a certain time, cooling and settling obtained reaction liquid to separate an oil phase and an aqueous phase (containing benzyl alcohol and hydrochloric acid), and performing crude distillation and rectification on the oil phase to obtain a benzyl alcohol finished product. According to the process, a continuous feeding and discharging manner is adopted for reaction, and a corresponding solvent and a corresponding catalyst are combined, so that the yield rate of the product is increased, and the productivity is improved.
Owner:HUBEI GREENHOME MATERIALS TECH INC
Halobutyl elastomers
The present invention relates to the modification of butyl elastomers, particularly halobutyl elastomers, under solvent free conditions with a phase transfer catalyst, particularly tetrabutyammonium bromide or trioctylmethylammonium chloride, in the presence of an alkyl metal salt of an oxygen or sulfur-based nucleophile. Suitable nucleophiles for use in the present invention include metal salts of tert-butylacetic acid, stearic acid, benzoic acid, 4-(dimethylamino)benzoic acid, antrancene-9-carboxylic acid, linoleic acid or mixture thereof, which are prepared by neutralization with an appropriate hydroxide base.
Owner:LANXESS INC
Preparation method of p-chlorophenylglycine
InactiveCN111470994AAvoid poisoningAvoid pollutionOrganic compound preparationCatalystsChlorobenzenePtru catalyst
The invention provides a preparation method of p-chlorophenylglycine, which comprises the following steps: adding a mixed solution of a chloroform solution, a catalyst and p-chlorobenzaldehyde into asodium hydroxide solution, mixing, and dropwisely adding liquid ammonia while stirring for 2-6 hours; after finishing dropwise adding the liquid ammonia, adding an ammonium bicarbonate solution, and reacting for 5-10 hours at room temperature; after the reaction is finished, distilling and concentrating a reaction solution, decolorizing and filtering by using activated carbon, adjusting the pH value of a filtrate to 6.0 by using an inorganic acid, cooling and filtering to obtain a crude product and mother liquor, washing the crude product by using water, ethanol and diethyl ether, and drying to obtain a finished product p-chlorophenylglycine; wherein the catalyst is trioctyl methyl ammonium chloride; wherein the molar ratio of the p-chlorobenzaldehyde to the trioctyl methyl ammonium chloride to the chloroform to the sodium hydroxide to the ammonium bicarbonate is 1.0 : (0.02-0.38) : (1.5-2.5) : (6.0-10.0) : (0.03-0.07). The synthesis process is simple, raw materials are easy to obtain,the reaction is milder, and toxicity and pollution of cyanide are avoided.
Owner:上海开荣化工科技有限公司
Synthetic method of 4-fluorobenzaldehyde
InactiveCN101353297ALow priceShort synthetic routeOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSulfolaneHexamethylphosphoramide
The invention relates to a synthetic method of p-flurobenzaldehyde, which belongs to the chemical and pharmaceutical field. In the synthetic method, the p-chlorobenzaldehyde and potassium fluoride react under the condition of a solvent and a catalyst at high temperature, wherein, the solvent is one of sulfolane, dimethyl sulfoxide, dimethylformamide, dimethylacetamide, hexamethyl phosphoramide, nitrobenzene and ortho-nitrotoluene; the catalyst is one of or the mixture of two or more than two of benzyl triethyl amine chloride, tetrabutylammonium bromide, hexadecyltrimethylammonium chloride, methyltrioctylammonium chloride, tetraphenylphosphonium bromide, methyltriphenylphosphonium bromide, benzyl triphenyl phosphonium bromide and polyethylene glycol dimethyl ether. Compared with the conventional preparation method, the synthetic method of the invention has the advantages of cheap raw materials, short synthetic route and little 'three wastes' discharge. As the amount of the catalyst used is reduced and the price of the solvent is low, the production cost of the p-flurobenzaldehyde is decreased.
Owner:王俊华
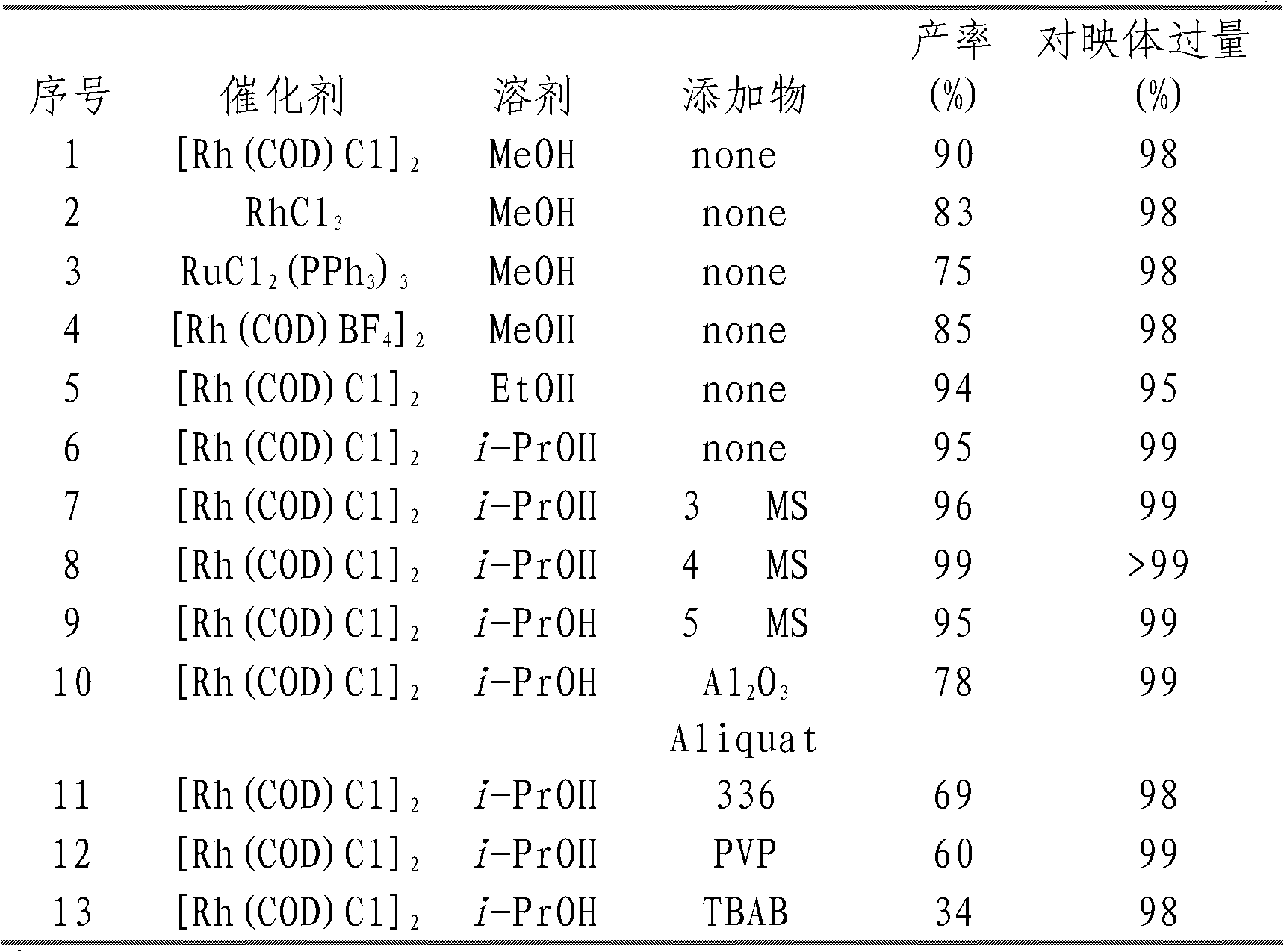
Method of compounding eight-hydrogen binaphthol derivative through rhodium catalytic hydrogenation
InactiveCN103130618AEasy to operateHigh regional selectivityOrganic chemistryOrganic compound preparationSolventTrioctylmethylammonium chloride
The invention discloses a method of compounding an eight-hydrogen binaphthol derivative through rhodium catalytic hydrogenation. A catalytic system used in the method is rhodium coordination compounds. A reaction is conducted under the conditions that temperatures are in a range of 0-100 DEG C, solvents are different alcohol, the pressure is in a range of (1-100) *1.01325*10<5>Pa, the ratio of zymolyte to catalysts is 100:1, metal precursor used is the rhodium coordination compounds, and additives used are different types of molecular sieves, alumina, aliquat 336, polyvinyl pyrrolidone (PVP) and tetrabutylammonium bromide. Hydrogenation is conducted on an optically pure binaphthol derivative to obtain the corresponding eight-hydrogen binaphthol derivative, wherein enantiomeric excess of the eight-hydrogen binaphthol derivative is kept to be larger than 99%. The method of compounding the eight-hydrogen binaphthol derivative through the rhodium catalytic hydrogenation is simple, convenient and practical to use, good in regioselectivity, and high in productivity. The reaction has green atom economy and is environment-friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN106984330AEvenly dopedImprove adsorption capacityCatalyst carriersWater contaminantsUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst and belongs to the technical fields of environment protection and chemical engineering catalysts. The preparation method comprises the following steps: by taking porous materials attapulgite, diopside, talc, trona, aluminum hydroxide and celestite as a carrier, after chambering and modifying the carrier through lithium hypochlorite and di(acetylacetone) beryllium, adding a surfactant methyl trioctyl ammonium chloride for surface activating treatment under the action of ultrasonic waves; then performing a hydrothermal reaction on the ultrasonic surface activated carrier in a hydrothermal reaction kettle with a compound mineralizer borax and potassium sulfate, catalytic active auxiliary agent precursors of tri(3-trifluoroacetyl-D-camphor) praseodymium (III), tricyclopentadiene promethium, terbium triacetate hydrate and holmium oxalate decahydrate rare earth metal organic compounds catalytic active central compound precursor common traditional metal organic compounds of ferrous fumarate and nickel citrate, and noble metal compounds of potassium argentite dithiocyanide (I) and a terpyridyl ruthenium chloride hexahydrate under the action of an emulsifier trimethylaminoglycerol glycerol stearate; and after drying a reaction product to remove water, firing the reaction product in a muffle furnace at a certain temperature to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
External use plaster used for easing pain and strengthening bone
ActiveCN107456548AIncrease stickinessImprove skin penetrationHydroxy compound active ingredientsAntipyreticAdjuvantAdditive ingredient
The invention belongs to the technical field of plaster preparations and particularly relates to an external use plaster used for easing pain and strengthening the bone. The external use plaster is prepared from a rhizoma kaempferiae fluid extract, musk, menthol, methyl salicylate, a leopard bone, chondroitin sulfate, borneol, diphenhydramine hydrochloride, camphor, zinc oxide, rosin, wool fat, vaseline, a transdermal enhancer, a tackifier, an increasing milk agent, and a rubber; the transdermal enhancer comprises a monarch drug and an adjuvant, wherein the monarch drug is combination of N-dimethylamino n-butyrate dodecyl ester and one of alpha-bisabolol, ferulic acid and glycerinum monopentyl ether, and the adjuvant is methyl trioctyl ammonium chloride or bromo-1-butyl-3-methyl-imidazole. The external use plaster has a very obvious effect of easing pain and strengthening the bone, has excellent transdermal absorptivity at the same time and takes effects quickly, the effective ingredients in the plaster do not volatilize easily, the viscidity of the plaster is good, and the quality is even. Meanwhile, the ingredients of the plaster are much easier to absorb by the skin after an emulsified slurry is made and gelatinized.
Owner:杭州仁德药业股份有限公司
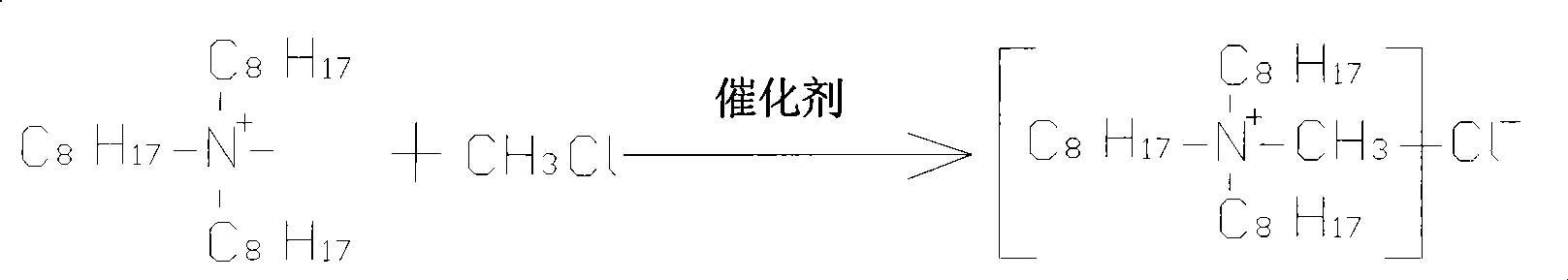
Production process of tri-n-octyl methyl ammonium chloride
InactiveCN101503362AImprove solubilityHigh selectivityFlotationProcess efficiency improvementSolubilityOrganic solvent
The invention discloses a technique for producing methyl trioctyl ammonium chloride, which comprises the steps: trioctylphosphine ammonium and methyl chloride react under the condition of catalyst existence to generate the methyl trioctyl ammonium chloride, and the methyl trioctyl ammonium chloride can be rapidly generated in the reaction. In addition, according to the characteristics of cation and surface activity which are specially possessed by the self of the methyl trioctyl ammonium chloride, the invention discloses the application of taking the methyl trioctyl ammonium chloride as collecting agent and metal extracting agent in raw mineral material selection, and the application proves that the methyl trioctyl ammonium chloride has the characteristics of higher solubility in organic solvent and water, stronger selectivity, insensitivity for alkaline medium, excellent stability, erosion resistance and no toxicity.
Owner:RUGAO WANLI CHEM IND
Synthesis method of 1-benzylpyridinium-3-carboxylate
ActiveCN103435541ANo pollution in the processHigh yieldOrganic chemistrySodium acetateSodium bicarbonate
The invention discloses a synthesis method of 1-benzylpyridinium-3-carboxylate. The 1-benzylpyridinium-3-carboxylate is obtained by performing a chemical reaction on benzyl chloride, nicotinic acid and sodium hydroxide serving as raw materials; fully dissolving the sodium hydroxide in water; adding the nicotinic acid; stirring to fully dissolve; adding a composite catalyst and a buffer; dropwise adding the benzyl chloride and continuously reacting for 1-2 hours after the dropwise adding is finished so as to obtain the 1-benzylpyridinium-3-carboxylate, wherein the composite catalyst is a mixture of K2CO3 or Na2CO3 and quaternary ammonium salt; the quaternary ammonium salt is one or more of tetrabutylammonium bromide, benzyltriethylammonium chloride, dodecyl trimethyl ammonium chloride, methyl trioctyl ammonium chloride, hexadecyl trimethyl ammonium bromide and bromogeramine; the buffer is one of sodium bicarbonate, sodium carbonate, sodium acetate and boric acid. The synthesis method has the advantages of high yield, short reaction time, low cost, high product purity, no environment pollution during production and environmental friendliness.
Owner:湖北吉和昌化工科技有限公司
Mild natural plant hair conditioner and preparation method thereof
ActiveCN109363978AEffective moisturizingPromote repairCosmetic preparationsHair cosmeticsAdditive ingredientTricholoma matsutake
The invention discloses a mild natural plant hair conditioner and a preparation method thereof. The mild natural plant hair conditioner comprises raw materials in parts by weight as follows: hexadecyltrimethylammonium chloride, dimethicone, trioctylmethylammonium chloride, Tricholoma matsutake extract, Matricaria recutita extract, Canarium album extract, a botanical fungicide, a plant conditionerand water, wherein the botanical fungicide is cortex lycii extract and / or Salix alba bark; the plant conditioner is fenugreek gum and / or locust bean gum. Compared with the prior art, the mild naturalplant hair conditioner is rich in various natural active components, mild, non-irritant, healthy and environmentally friendly, moistens scalp and hair effectively, is beneficial to repair of damaged hair, prevents the hair from getting split and dry and has good hair conditioning and protecting functions.
Owner:汕头市润晶化妆品有限公司
Preparation method of lambda-cyhalothrin
InactiveCN103420872AHigh crude oil contentHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationIsomerizationDistillation
The invention discloses a preparation method of lambda-cyhalothrin. The preparation method of the lambda-cyhalothrin comprises the steps that 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid serves as an initial raw material, DMF serves as a catalyst, n-hexane serves as solvent, an n-hexane solution of 3-(2,2-Dichlorovinyl)-2,2-dimethylcyclopropanecarbonyl chloride is obtained, a reaction among the n-hexane solution of the 3-(2,2-Dichlorovinyl)-2,2-dimethylcyclopropanecarbonyl chloride, 3-phenoxy-4-fluoro-benzaldehyde and sodium cyanide is carried out, methyl trioctyl ammonium chloride serves as a catalyst, a condensation reaction is carried out to obtain a cyhalothrin condensation compound, washing and desalting are carried out on the cyhalothrin condensation compound to obtain a cyhalothrin n-hexane solution, a composite catalyst is directly added to the cyhalothrin n-hexane solution, and an epimerization reaction is carried out to obtain the lambda-cyhalothrin. Compared with the prior art, the technological process is simple, the solvent does not need to be replaced in the process of preparation, the situation that isopropanol is used for carrying out working procedures such as rectification and dewatering is avoided, and the same solvent is adopted; due to the fact that the composite catalyst is adopted, the rate of the epimerization reaction is improved, and due to the facts that the n-hexane serves as epimerization solvent, and the isopropanol is not adopted, the working procedure of distillation recycling of the isopropanol and the working procedure of dewatering of the isopropanol are omitted, material loss is reduced, production cost is reduced, industrial production can be easily carried out, and popularization prospect and application prospect are wide.
Owner:LIANYUNGANG CCA CHEM CO LTD
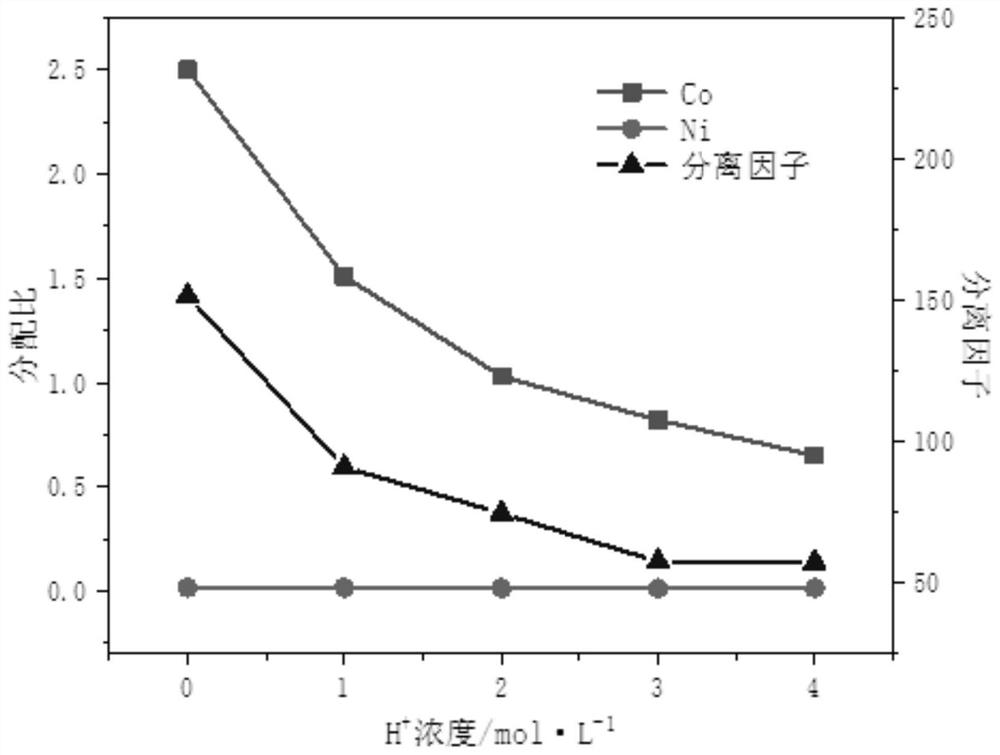
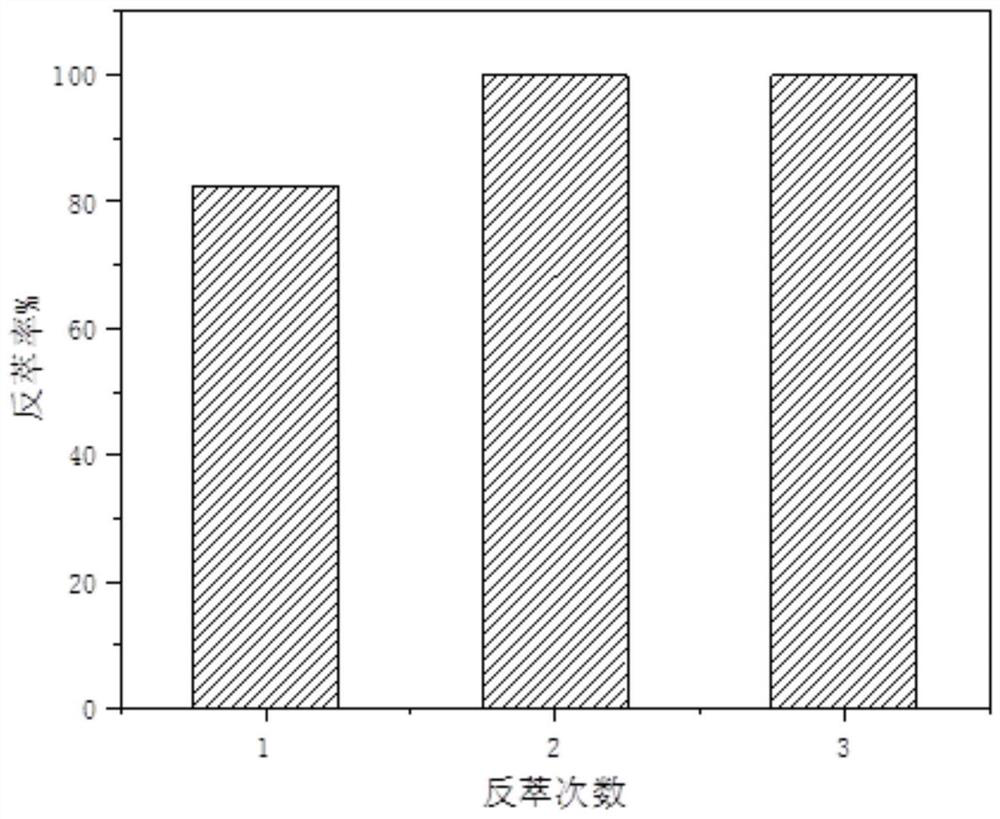
Hydrophobic deep-eutectic solvent used for separating nickel and cobalt ions, preparation method of hydrophobic deep-eutectic solvent and method for separating nickel and cobalt ions
ActiveCN112981139ALarge extraction capacityReduce usageProcess efficiency improvementSolventAmmonium bromide
The invention discloses a hydrophobic deep-eutectic solvent used for separating nickel and cobalt ions, a preparation method of the hydrophobic deep-eutectic solvent and a method for separating the nickel and cobalt ions. In the hydrophobic deep-eutectic solvent, the molar ratio of a hydrogen bond donor to a hydrogen bond acceptor is (1: 1)-(1: 1.5); the hydrogen bond acceptor adopts trioctyl methyl ammonium chloride, and the hydrogen bond donor adopts menthol; or the hydrogen bond acceptor adopts trioctyl methyl ammonium bromide, and the hydrogen bond donor adopts menthol; or the hydrogen bond acceptor adopts trioctyl methyl ammonium bromide, and the hydrogen bond donor adopts thymol. The method for separating the nickel and cobalt ions comprises the following steps that a nickel-cobalt mixed solution is mixed with the hydrophobic deep-eutectic solvent, centrifugal phase separation is carried out after extraction equilibrium to obtain a cobalt-containing organic phase, cobalt in the organic phase is reversely extracted into a water phase by adopting a sodium sulfate water solution, and meanwhile, the hydrophobic deep-eutectic solvent is reused. The method is not easily influenced by acidity during nickel and cobalt ion separation, meanwhile, the extraction capacity is high, and reverse extraction is easy.
Owner:XI'AN UNIVERSITY OF ARCHITECTURE AND TECHNOLOGY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107008420AEvenly dopedEnhanced anti-toxicityCatalyst carriersOther chemical processesUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, belonging to the technical field of environment-friendly and chemical catalysts. The preparation method comprises the following steps: by taking attapulgite, diopside, illite, ulexite, aluminum hydroxide and celestine porous materials as carriers, performing pore expansion and modification to the carriers through lithium hypochlorite and beryllium bis(acetylacetonate), adding a surfactant methyl trioctylammonium chloride and performing surface activation treatment under ultrasonic wave effect, then leading the ultrasonically surface-activated carriers to have hydrothermal reaction with a complex mineralizer, namely borax and potassium sulfate, catalytic activity assistant precursors, namely praseodymium(III) tris[3-(trifluoromethylhydroxymethylene)-D-camphorate], promethium tricyclopentadienide, tris(4,4,4-trifluoro-1-(2-thienyl)-1,3-butanediono) europium and lutetium carbonate hydrate rare earth metal organic compound, and catalytic active site component precursors, namely common transitional metal organic compound ferrous fumarate, nickel citrate and tungsten catechol ethylenediamine complex and precious metal compound potassium bis(thiocyanate) argentate (I) in a hydrothermal reactor under the action of an emulsifier dodecyl dimethyl (2-hydroxyl) ethylamine chloride, drying reactive products to remove moisture, and firing in a muffle furnace at certain temperature, to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
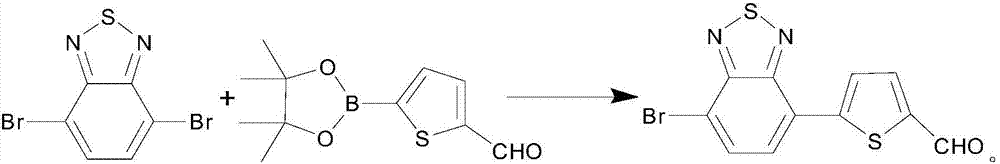
Asymmetric synthesis method of 4,7-dibromo-2,1,3-diazosulfide symmetric structure
InactiveCN107964010AImprove conversion rateEasy to operateOrganic chemistrySynthesis methodsPalladium catalyst
The invention discloses an asymmetric synthesis method of a 4,7-dibromo-2,1,3-diazosulfide symmetric structure. The purpose of optimizing conditions of coupling reaction of 4,7-dibromo-2,1,3- diazosulfide C-C coupling reaction is achieved, on the basis of Suziki reaction, THF / H2O is used as a solvent system, Pd(PPh3)4 or Pd(dppf)Cl2 is used as a palladium catalyst, a phase transfer catalyst whichis methyl trioctyl ammonium chloride and the like is introduced, reaction conditions are mild, moreover, the reaction time is shortened, and the percent conversion of a reactant is increased. The synthesis method is simple to operate, the compatibility of a substrate is good, and therefore, the method can be applied to synthesis of organic compounds with similar structures well.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
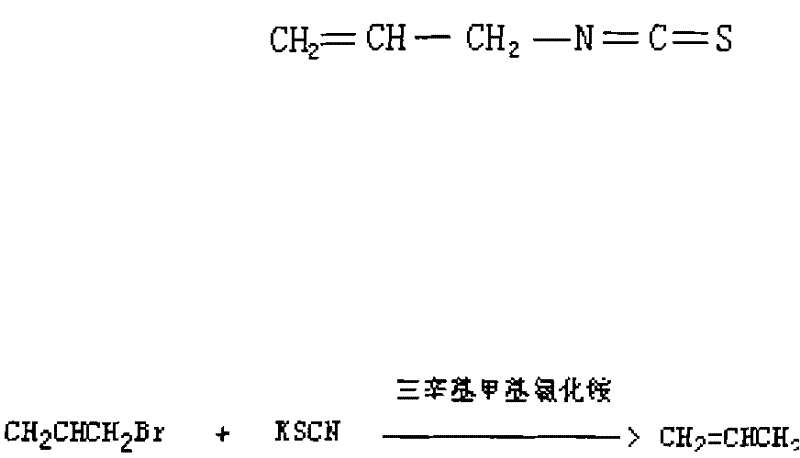
Method for synthesizing high-content allyl isothiocyanate
The invention discloses a method for synthesizing high-content allyl isothiocyanate. The allyl isothiocyanate has bactericidal activity, and can be taken as a fumigant instead of curafume. In the method, methyl trioctyl ammonium chloride is taken as a phase transfer catalyst for use in an allyl isothiocyanate synthesis reaction, so that reaction time is shortened to 1.4 hours, and the reaction yield is increased by 98.9 percent; and acetonitrile is taken as a reaction solvent, and allyl bromide reacts with potassium rhodanate, so that allyl isothiocyanate of which the content is over 98 percent is finally synthesized.
Owner:徐波勇 +2
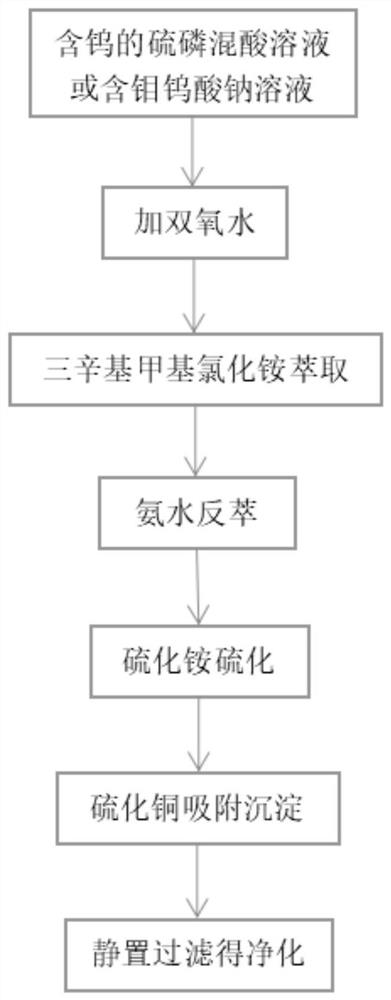
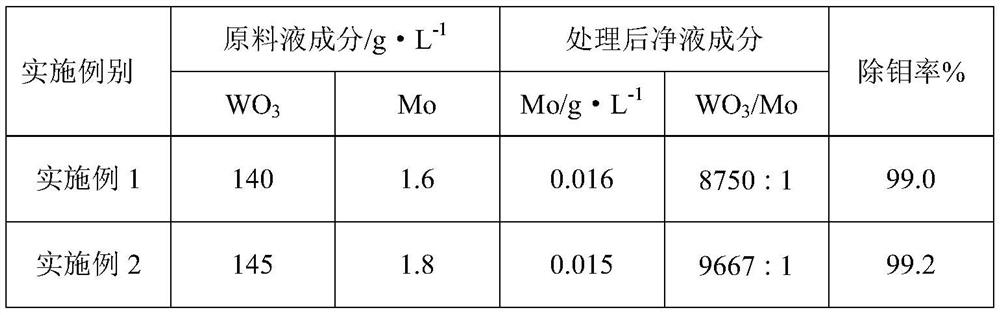
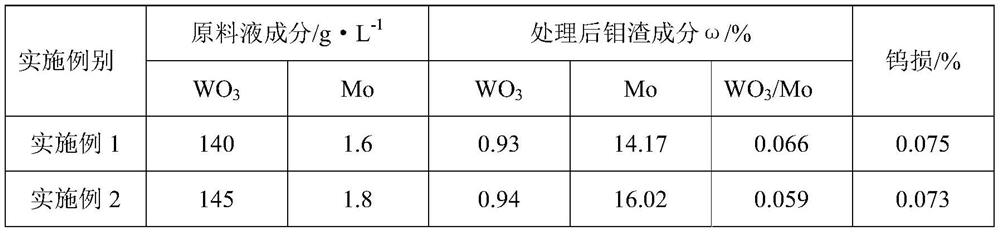
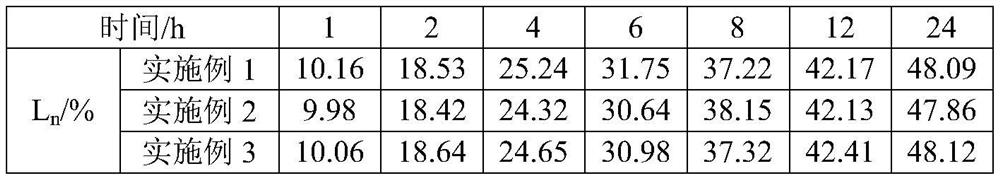
Method for efficiently removing molybdenum based on extraction-precipitation combination
PendingCN113621835AMolybdenum impurity content is not limitedWide adaptabilityProcess efficiency improvementHigh concentrationO-Phosphoric Acid
The invention discloses a method for efficiently removing molybdenum based on extraction-precipitation combination. The method comprises the following steps that an extraction organic phase is formed by using methyl trioctyl ammonium chloride as an extraction agent, tributyl phosphate as a cosolvent and sulfonated kerosene as a diluent, and meanwhile, the organic phase extraction agent is transformed, so that tungsten in an acid system can be extracted, or the tungsten in a molybdenum-containing sodium tungstate solution can be extracted, the separated sulfuric acid and phosphoric acid are returned for use, the negative tungsten organic phase is subjected to reverse extraction with ammonia water, a high-concentration ammonium tungstate solution can be obtained, and cation transformation is achieved. According to the method provided by the invention, the content of impurity molybdenum can be effectively removed.
Owner:FUJIAN JINXIN TUNGSTEN
Ozone heterogeneous oxidation solid catalyst preparation method
InactiveCN107051527AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionPalmitates
The invention belongs to the technical field of environment protection and chemical catalysts and relates to an ozone heterogeneous oxidation solid catalyst preparation method. The preparation method includes: taking porous mineral materials including attapulgite, diopside, talc, trona, amazonite and kunzite as carriers; subjecting the carriers to lithium hypochlorite and bis(acetylacetone)beryllium broaching modification; adding surfactant trioctylmethyl ammonium chloride for surface activation under the action of ultrasonic waves; subjecting the carriers to hydrothermal reaction, with a complex mineralizer composed of borax and potassium sulfate, catalytic activity auxiliary agent precursors including tri(3-trifluoroacetyl-D-camphor)praseodymium (III), promethium tricyclopentadiene, terbium acetate hydrate and thulium trifluoromethanesulfonate (III) and catalytic activity central component precursors including ferrous fumarate, nickel citrate, potassium dithiocyanoargentate (I) and tetraammine dichloropalladium, in a hydrothermal reactor under the action of trimethylamino glycolate ammonium iodide palmitate serving as an emulsifying agent; drying to remove moisture, and firing in a muffle furnace at a certain temperature to obtain an ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107029743AEvenly dopedImprove adsorption capacityCatalyst carriersOther chemical processesUltrasound - actionSodium Bentonite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, and belongs to the technical field of environmental protection and chemical catalysts. The preparation method includes the steps: taking attapulgite, diopside, bentonite, polyhalite, fly ash and coal gangue as carriers; broaching the carriers by lithium hypochlorite and beryllium bis(acetylacetonate); adding the surfactant trioctylmethylammonium chloride to perform activating under the action of ultrasonic wave; performing hydrothermal reaction on the carriers with composite mineralizing agents including borax and potassium sulfate, catalytic active aid precursors including praseodymium tri(3-trifluoroacetyl-D-camphor) (III), neodymium 1,1,1-trifluoroacetyl acetone, thulium trifluoromethanesulfonate (III) and lutetium carbonate hydrate, and catalytic active center precursors including ferrous fumarate, nickel citrate, zinc lactate and iridium dihydrate tetrachloride in a hydrothermal reactor under the action of the emulsifier, N-hexadecyldimethyl-N'-trimethyl-propyl ammonium dichloride; drying for removing water and firing in a muffle furnace to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107008436AEvenly dopedImprove adsorption capacityOther chemical processesWater treatment compoundsUltrasound - actionRare earth metal compounds
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, belonging to the technical field of environment-friendly and chemical catalysts. The preparation method comprises the following steps: by taking attapulgite, diopside, illite, ulexite, pulverized fuel ash and coal gangue porous materials as carriers, performing pore expansion and modification to the carriers through lithium hypochlorite and beryllium bis(acetylacetonate), adding a surfactant methyl trioctylammonium chloride and performing surface activation treatment under ultrasonic wave effect, then leading the ultrasonically surface-activated carriers to have hydrothermal reaction with a complex mineralizer: borax and potassium sulfate, catalytic activity assistant precursors: praseodymium(III) tris[3-(trifluoromethylhydroxymethylene)-D-camphorate], promethium tricyclopentadienide, tetra(2,2,6,6-tetramethyl-3,5-heptanedionato)cerium(IV) and holmium oxalate rare earth metal organic compound, and catalytic active site component precursors: common transitional metal organic compound ferrous fumarate, nickel citrate and tungsten catechol ethylenediamine complex and precious metal compound trisodium hexanitrosorhodium in a hydrothermal reactor under the action of an emulsifier lauramidopropyl trimethylammonium methyl sulfate, drying reactive products to remove moisture, and firing in a muffle furnace at certain temperature, to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107029746AImprove bindingHigh activityCatalyst carriersOther chemical processesUltrasound - actionPotassium thiocyanate
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, and belongs to the technical field of environmental protection and chemical catalysts. The preparation method includes the steps: taking attapulgite, diopside, steatite, trona, coal ash and coal gangue porous materials as carriers; chambering and modifying the carriers by lithium hypochlorite and bis(acetylacetonato) beryllium; adding methyl trioctyl ammonium chloride serving as a surface active agent for surface activation treatment under the action of ultrasonic waves; performing hydrothermal reaction on the ultrasonic surface active carriers with compound mineralizing agents including borax and potassium sulfate, catalytic active auxiliary precursors including tri(3-trifluoroacetyl-D-camphor) praseodymium (III), tricyclic pentadiene promethium, terbium acetate hydrate and tri(fluoroform sulfimide) ytterbium rare-earth metal organic compounds and catalytic active central component precursors including ordinary transition metal organic compound ferrous fumarate, nickel citrate, precious metal compound K[Ag(SCN)2] and dipotassium hexachloroosmate in a hydrothermal reactor under the action of trimethylamine laurate ammonium chloride glycolate serving as an emulsifier; drying a reaction product, removing water and firing the reaction product in a muffle furnace at a certain temperature to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107008435AEvenly dopedImprove adsorption capacityOther chemical processesWater treatment compoundsUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, belonging to the technical field of environment-friendly and chemical catalysts. The preparation method comprises the following steps: by taking attapulgite, diopside, steatite, boron tribromide, brucite and serpentinite porous materials as carriers, performing pore expansion and modification to the carriers through lithium hypochlorite and beryllium bis(acetylacetonate), adding a surfactant methyl trioctyl ammonium chloride and performing surface activation treatment under ultrasonic wave effect, then leading the ultrasonically surface-activated carriers to have hydrothermal reaction with a complex mineralizer (borax and potassium sulfate), catalytic activity assistant precursors, namely praseodymium(III) tris[3-(trifluoromethylhydroxymethylene)-D-camphorate], promethium tricyclopentadienide, tris(2,2,6,6-tetramethyl-3,5-heptanedionato)gadolinium(III), and lutetium carbonate hydrate rare earth metal organic compound, and catalytic active site component precursors, namely common transitional metal organic compound ferrous fumarate, nickel citrate and tungsten catechol ethylenediamine complex and precious metal compound dichlorodiamminoplatinum in a hydrothermal reactor under the action of an emulsifier dimethylaminoacrylate laurate ammonium chloroacetate, drying reactive products to remove moisture, and firing in a muffle furnace at certain temperature, to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
An external plaster for analgesia and bone strengthening
ActiveCN107456548BObvious analgesic effectEfficient transdermal absorption rateHydroxy compound active ingredientsAntipyreticAdjuvantGlycerol
The invention belongs to the technical field of plaster preparations, and in particular relates to an external plaster for analgesia and bone strengthening, which consists of kaempferia liquid extract, musk, menthol, methyl salicylate, leopard bone, chondroitin sulfate, borneol, Diphenhydramine hydrochloride, camphor, zinc oxide, rosin, lanolin, vaseline, transdermal enhancer, thickener, emulsifier, rubber, transdermal enhancer includes main agent and auxiliary agent, of which the main agent is N Combination of lauryl dimethylamino-n-butyrate with any one of α-bisabolyl alcohol, ferulic acid and glycerol monopentyl ether, the auxiliary agent is trioctylmethyl ammonium chloride or 1-bromide Butyl-3-methyl-imidazole. The plaster for external use of the invention has very obvious analgesic and bone-strengthening effects, and at the same time has good transdermal absorption rate, quick effect, effective ingredients in the plaster are not easy to volatilize, and the plaster has good viscosity and uniform quality. At the same time, it is made into an emulsified slurry and then coated with glue to make the ingredients of the external plaster more easily absorbed by the skin.
Owner:杭州仁德药业股份有限公司
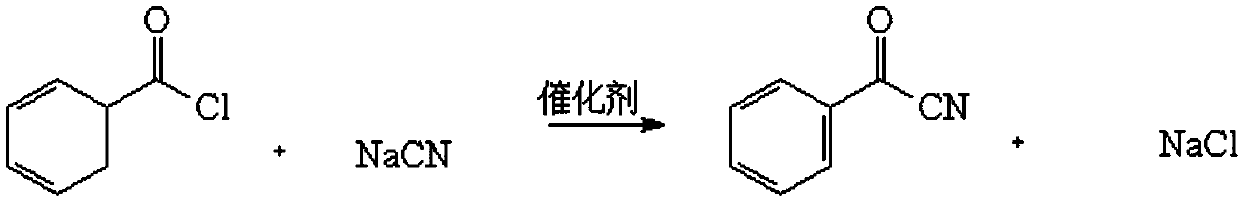
Synthesis method of benzoyl cyanide
InactiveCN109651193AEasy to operateReduce pollutionPreparation by cyanide reactionSynthesis methodsGlycerol
The invention discloses a synthesis method of benzoyl cyanide. The method is characterized in that sodium cyanide and benzoyl chloride are taken as raw materials, the mixture of polyethylene glycol 600 and trioctylmethylammonium chloride is taken as a phase transferring catalyst, water or glycerol is taken as an auxiliary catalyst, the reaction with the benzoyl chloride is conducted at the temperature of 60 DEG C-110 DEG C, a benzoyl cyanide coarse product and a reaction bi-product are prepared after the reaction is conducted for 3-5 hours, and the coarse product is washed and separated to prepare a benzoyl cyanide fine product under the action of a solvent. The method has the advantages that the benzoyl cyanide is synthesized through a simple method, an alkaline chlorination method is adopted to perform treatment in order to reduce pollution and turn harm into benefit, and therefore cyanide is oxidized and destructed by active chlorine under strong alkaline conditions to generate atoxic sodium chloride; the solvent can be recycled, so that the synthesis method has the advantages of low pollution, high conversion, simple operation and the like.
Owner:江苏佳麦化工有限公司
Ozone heterogeneous oxidation solid catalyst preparation method
InactiveCN107051514AImprove adsorption capacityImprove bindingCatalyst carriersWater contaminantsUltrasound - actionSodium Bentonite
The invention belongs to the technical field of environment protection and chemical catalysts and relates to an ozone heterogeneous oxidation solid catalyst preparation method. The preparation method includes: taking porous mineral materials including attapulgite, diopside, bentonite, polyhalite, nitratine and dolomite as carriers; subjecting the carriers to lithium hypochlorite and bis(acetylacetone)beryllium broaching modification; adding surfactant trioctylmethyl ammonium chloride for activation under the action of ultrasonic waves; subjecting the carriers to hydrothermal reaction, with a complex mineralizer composed of borax and potassium sulfate, catalytic activity auxiliary agent precursors including tri(3-trifluoroacetyl-D-camphor)praseodymium (III), 1,1,1-neodymium trifluoroacetylacetonate, tris[N,N-bis(trimethylsilane)amine]erbium and lutetium carbonate hydrate and catalytic activity central precursors including ferrous fumarate, nickel citrate, zinc lactate and tetraammine dichloropalladium, in a hydrothermal reactor under the action of N-dimethyldodecyl-N'-dodecyl-dimethyl-2-hydroxypropyl ammonium chloride serving as an emulsifying agent; drying to remove moisture, and firing in a muffle furnace at a certain temperature to obtain an ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone non-homogeneous oxidation solid catalyst
InactiveCN107138166AImprove adsorption capacityImprove bindingCatalyst carriersWater contaminantsUltrasound - actionSodium Bentonite
The invention relates to a preparation method of an ozone non-homogeneous oxidation solid catalyst and belongs to the technical field of environmental protection and chemical catalysts. The preparation method comprises the following steps: taking attapulgite, diopside, bentonite, polyhalite, brucite and a serpentine porous material as carriers; adding surfactant trioctylmethylammonium chloride for surface activation treatment under the ultrasonic action after the carriers are subjected to lithium hypochlorite and bis (acetylacetone) beryllium reaming and modification; then enabling the ultrasonic surface activation carriers to perform hydrothermal reaction with compound mineralizer borax and potassium sulfate, catalytic activity auxiliary precursor tris(3-trifluoroacetyl-D-camphor) praseodymium (III), 1,1,1-trifluoroacetylacetone neodymium, holmium oxalate, a lutetium carbonate hydrate rare earth metal organic compound, catalytically-active central component precursor general transition metal organic compound ferrous fumarate, nickel citrate, zinc lactate and precious metal compound tetrachlorate potassium in a hydrothermal reactor under the action of emulsifier tri-cetylmethyl ammonium chloride; drying a reaction product, removing moisture and then firing at a certain temperature in a muffle furnace to obtain the ozone non-homogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
Preparation method of ozone heterogeneous oxidation solid catalyst
InactiveCN107008437AEvenly dopedImprove adsorption capacityOther chemical processesWater treatment compoundsUltrasound - actionLithium hypochlorite
The invention relates to a preparation method of an ozone heterogeneous oxidation solid catalyst, belonging to the technical field of an environment-friendly and chemical catalyst. The preparation method comprises the following steps: by taking attapulgite, diopside, illite, ulexite, agalmatolite and boron tribromide porous materials as carriers, performing pore expansion and modification to the carriers through lithium hypochlorite and beryllium bis(acetylacetonate), adding a surfactant methyl trioctylammonium chloride and performing surface activation treatment under ultrasonic wave effect, then leading the ultrasonically surface-activated carriers to have hydrothermal reaction with a complex mineralizer, namely borax and potassium sulfate, catalytic activity assistant precursors, namely praseodymium(III) tris[3-(trifluoromethylhydroxymethylene)-D-camphorate], promethium tricyclopentadienide, tris(4,4,4-trifluoro-1-(2-thienyl)-1,3-butanediono) europium and terbium(III) acetate hydrate rare earth metal organic compound, and catalytic active site component precursors, namely common transitional metal organic compound ferrous fumarate, nickel citrate and zirconium ammonium carbonate and precious metal compound tris(bipyridine)ruthenium(ii)chloride hexahydrate in a hydrothermal reactor under the action of an emulsifier dihydroxyethyl octadecylammonium methosulfate, drying reactive products to remove moisture, and firing in a muffle furnace at certain temperature, to obtain the ozone heterogeneous oxidation solid catalyst.
Owner:SICHUAN NORMAL UNIVERSITY
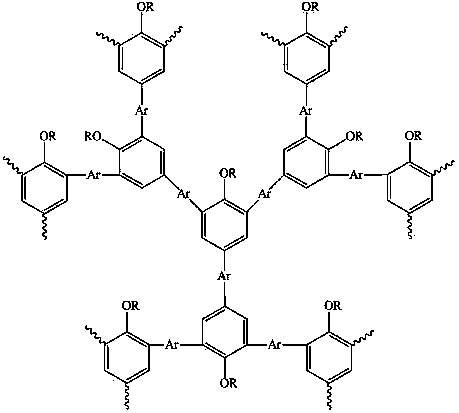
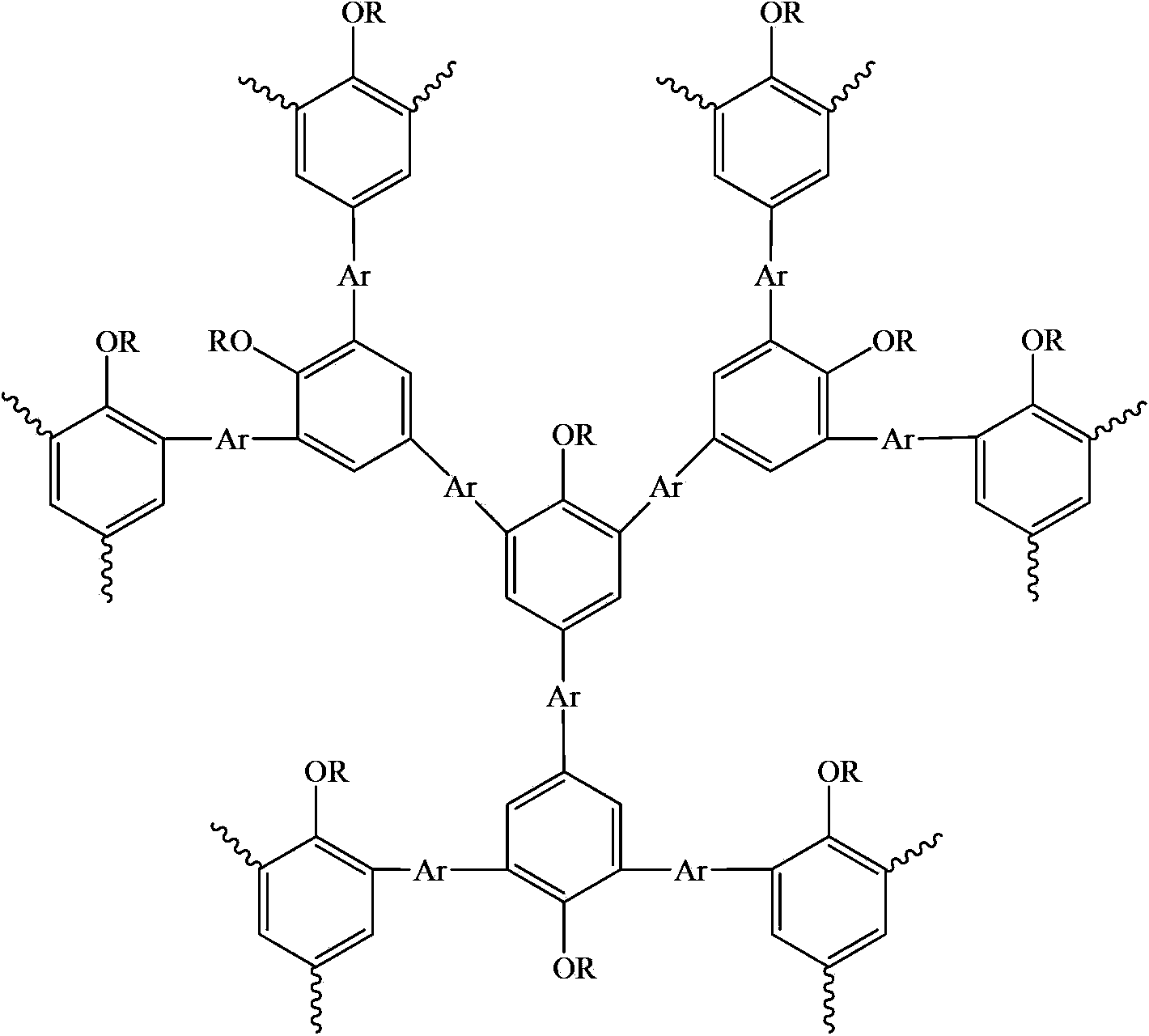
Hyperbranched polymer with 2,4,6-tribromo-1-alkoxybenzene as matrix and preparation method thereof
InactiveCN103172834BImprove film formationEasy to processEther preparation by ester reactionsFluorescenceTrioctylmethylammonium chloride
The invention relates to a hyperbranched polymer with 2,4,6-tribromo-1-alkoxybenzene as a matrix and a preparation method thereof. The hyperbranched polymer has the general formula shown in the specification. The preparation process comprises the following steps of: taking borate of arylidene and 2,4,6-tribromo-1-alkoxybenzene, then taking a catalyst and a phase transfer agent trioctyl methyl ammonium chloride, adding the materials to a flask containing methyl benzene, raising the temperature to carry out reflux, then adding borate of arylidene again to cap the end, carrying out reflux, adding bromobenzene and then carrying out reflux to cap the end to obtain mixed liquor; and then precipitating the mixed liquor with methanol, after filtering and drying, carrying out column passing and purification on the precipitate with methyl benzene as an eluent, precipitating the liquor with methanol after concentration and then filtering and drying the precipitate, thus obtaining the hyperbranched polymer. The hyperbranched polymer and the preparation method have the advantages that the hyperbranched polymer can be well dissolved in an organic solvent, is convenient for forming films, has good processability, can emit stronger fluorescence and becomes a promising OLED (organic light emitting diode) material; and the polymerization method is simple to operate, is feasible and is higher in yield.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Dental material including propylbarbituric acid polymerization catalyst
ActiveUS20160175203A1Improve curing effectOvercomes the problem about discolorability of a dental materialImpression capsOrganic-compounds/hydrides/coordination-complexes catalystsTrioctylmethylammonium chlorideChemistry
Owner:SHOFU INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com