Asymmetric synthesis method of 4,7-dibromo-2,1,3-diazosulfide symmetric structure
A technology of benzothiadiazole and synthesis method, applied in the direction of organic chemistry, can solve the problems of long reaction time and low reaction yield, achieve the effects of good substrate compatibility, reduce reaction time, and increase conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Analytical pure tetrahydrofuran (THF) and H 2 O is mixed in a volume ratio of 2:1.
[0019] (2) Measure 100 mL of the product obtained in step (1) into a two-necked flask, heat to boiling, and cool to room temperature.
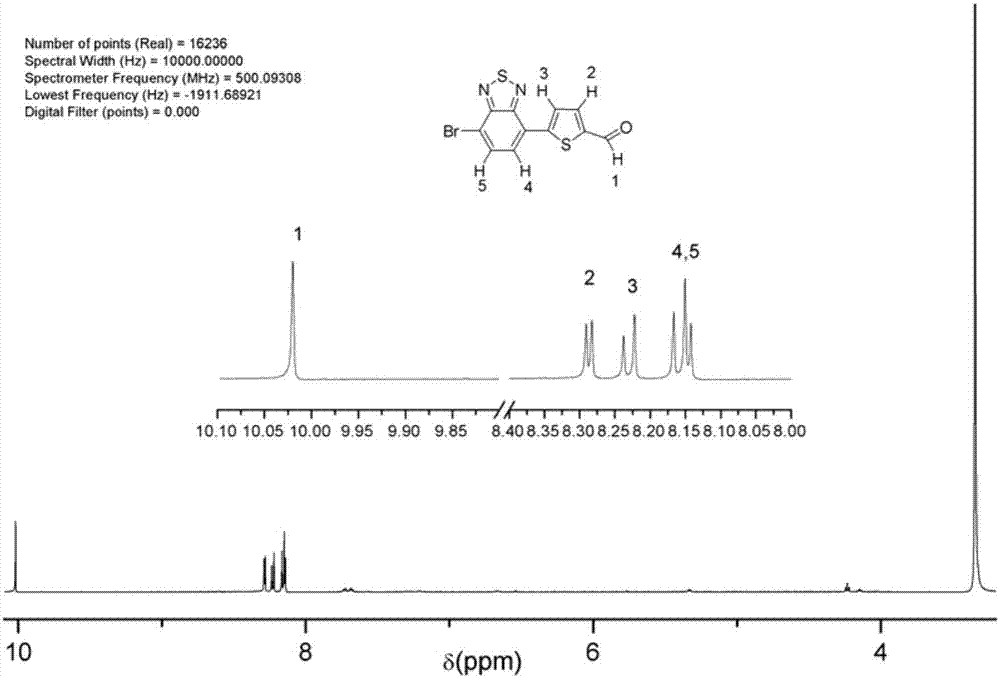
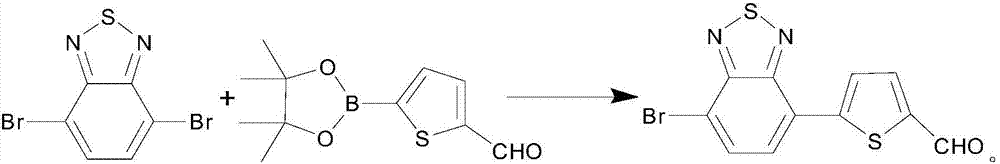
[0020] (3) Weigh 1.617g (5.5mmoL) 4,7-dibromo-2,1,3-benzothiadiazole, 0.7800g (5mmoL) 5-formyl-2-thiophene boronic acid, 1.47g (40mmoL) Potassium carbonate was added to the product obtained in step (2) and stirred for dissolution, under argon protection.
[0021] (4) adding 0.25g mass percent concentration in the product obtained in step (3) is 5% Pd (PPh 3 ) 4 and 0.05mL of analytically pure trioctylmethylammonium chloride, reflux at 90°C, track the reaction with thin-layer chromatography until the concentration of the reactant is basically unchanged, and stop the reaction.
[0022] (5) post-treatment: cooling in the product obtained in step (4), spin-dried, dissolved with analytically pure dichloromethane (DCM), washed with water, extracted, c...
Embodiment 2
[0024] (1) Analytical pure tetrahydrofuran (THF) and H 2 O is mixed in a volume ratio of 2:1.
[0025] (2) Measure 100 mL of the product obtained in step (1) into a 250 mL two-neck flask, heat to boiling, and cool to room temperature.
[0026] (3) Weigh 1.617g (5.5mmoL) 4,7-dibromo-2,1,3-benzothiadiazole, 0.7800g (5mmoL) 5-formyl-2-thiophene boronic acid and 1.47g (40mmoL) Potassium carbonate was added to the product obtained in step (2) and stirred for dissolution, under argon protection.
[0027] (4) adding 0.2042g mass percent concentration in the product obtained in step (3) is 5% Pd(dppf)Cl 2 , reflux at 90°C, track the reaction with thin-layer chromatography until the concentration of the reactant is basically unchanged, and stop the reaction.
[0028] (5) Aftertreatment: the product gained in step (4) is cooled, spin-dried, dissolved with analytically pure dichloromethane (DCM), washed with water, extracted, and the organic phase is collected, spin-dried, and separat...
Embodiment 3
[0030] (1) Analytical pure tetrahydrofuran (THF) and H 2 O is mixed in a volume ratio of 2:1.
[0031] (2) Measure 100 mL of the product obtained in step (1) into a 250 mL two-neck flask, heat to boiling, and cool to room temperature.
[0032] (3) Weigh 1.617g (5.5mmoL) 4,7-dibromo-2,1,3-benzothiadiazole, 0.7800g (5mmoL) 5-formyl-2-thiophene boronic acid and 1.47g (40mmoL) Potassium carbonate was added to the product obtained in step (2) and stirred for dissolution, under argon protection.
[0033] (4) adding 0.2042g mass percent concentration in the product obtained in step (3) is 5% Pd(dppf)Cl 2 and 0.05mL of analytically pure trioctylmethylammonium chloride, reflux at 90°C, track the reaction with thin-layer chromatography until the concentration of the reactant is basically unchanged, and stop the reaction.
[0034] (5) post-processing: the product obtained in step (4) is cooled, spin-dried, dissolved with analytically pure dichloromethane (DCM), washed with water, extr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com