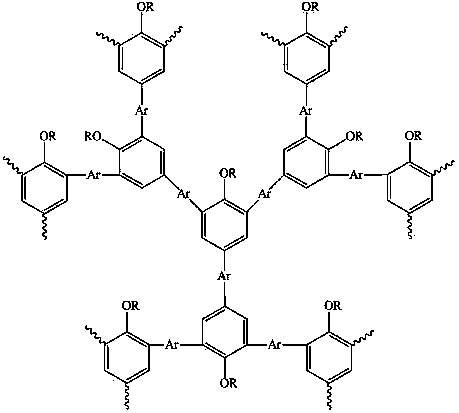
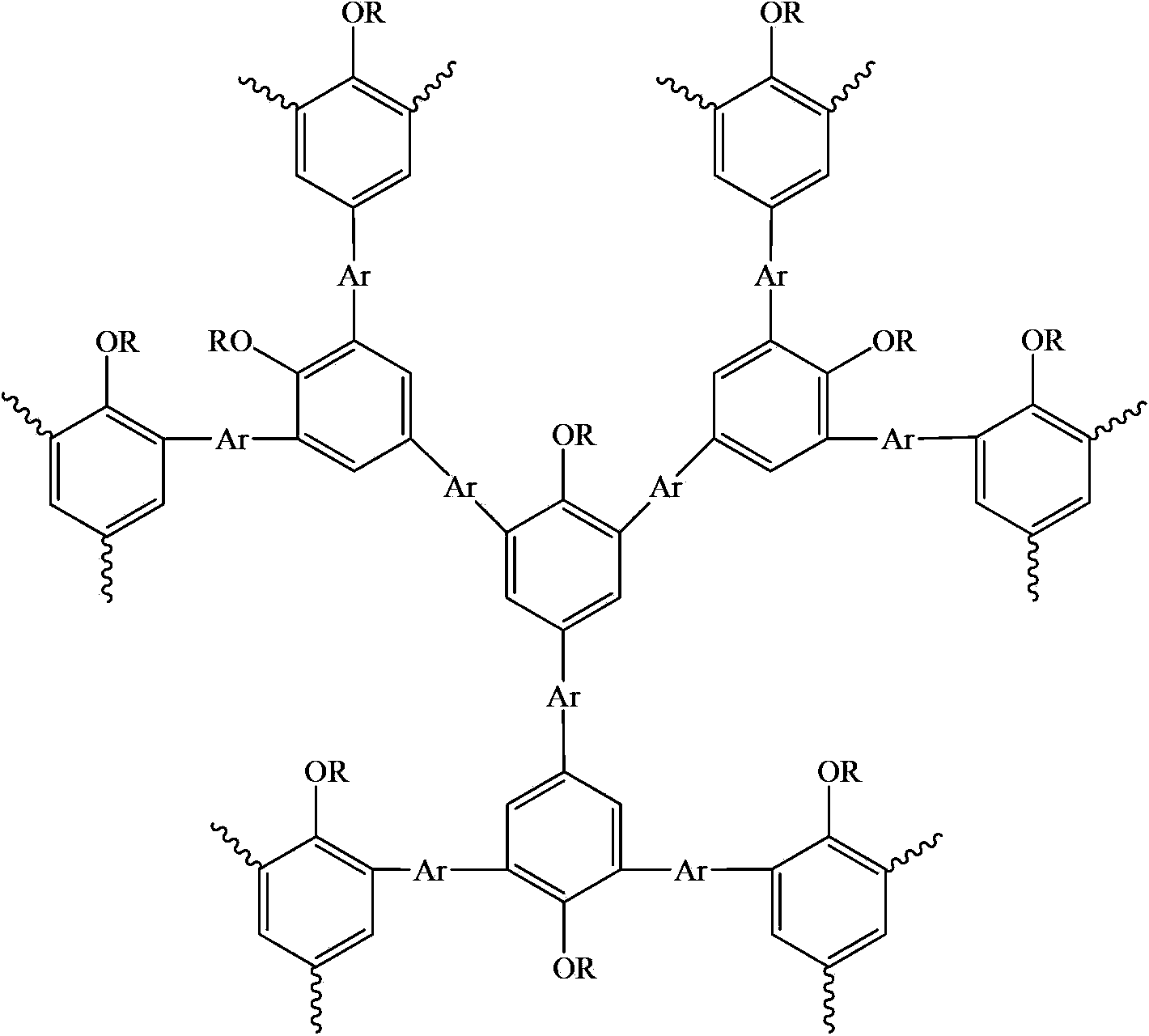
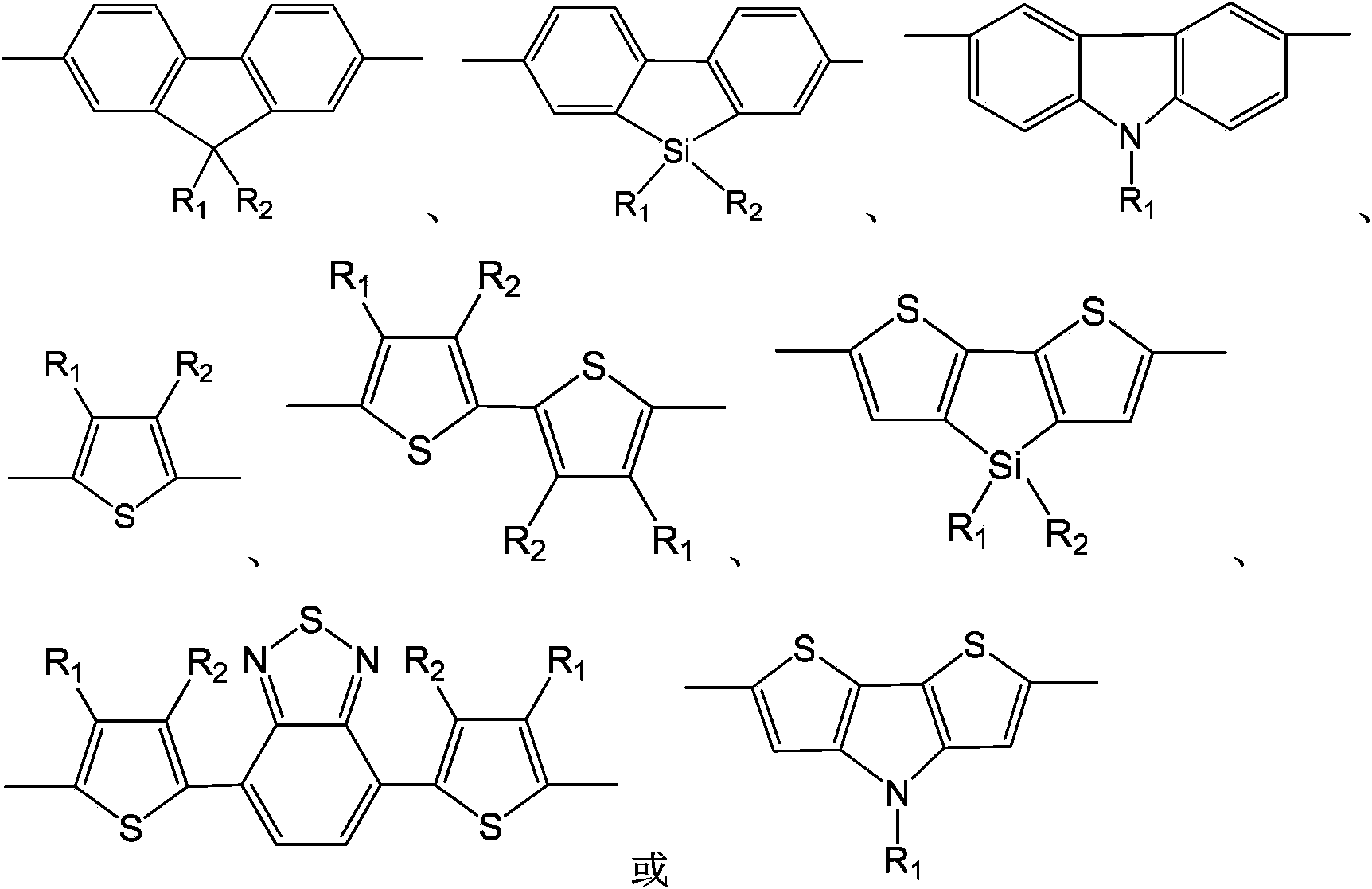
Hyperbranched polymer with 2,4,6-tribromo-1-alkoxybenzene as matrix and preparation method thereof
A technology of hyperbranched polymer and alkoxybenzene, which is applied in the directions of ether preparation, ester reaction preparation of ether, organic chemistry, etc., and achieves the effects of high yield, good processing performance, and simple and feasible operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of the hyperbranched polymer of 2,4,6-tribromo-1-octyloxybenzene and 9-hexylcarbazole, the reaction formula is as follows:
[0025]
[0026] The specific synthesis process is as follows:
[0027] i. Synthesis of 2,4,6-tribromo-1-octyloxybenzene
[0028] Add 5mmol (1.654g) 2,4,6-tribromophenol and 6~7ml methanol into a 50ml double-necked flask, stir; add 10mmol (0.400g) NaOH after dissolving, and heat up to 50°C; after reflux for 1h, dropwise Inject 1.728g bromooctane (10mmol, 99% analytically pure), and reflux for 24h. When a white solid appears at the bottom of the flask, add a small amount of methanol to redissolve it. After reflux for 24 hours, extract the product with chloroform. After evaporating the solvent, use n-hexane as the eluent for column purification, and dry the product in a vacuum oven. Finally, 1.552 g of colorless transparent liquid 2,4,6-tribromo-1-octyloxybenzene was obtained, yield: 70.6%.
[0029] ii. Synthesis of 2,4,6-tribro...
Embodiment 2
[0035] The preparation of 2,4,6-tribromo-1-octyloxybenzene and 9,9-dioctylfluorene hyperbranched polymer, the reaction formula is as follows:
[0036]
[0037] The specific synthesis process is as follows:
[0038] The preparation process of 2,4,6-tribromophenoxy n-octane is the same as in Example 1;
[0039] Take 0.2mmol (88mg) 2,4,6-tribromo-1-octyloxybenzene, 0.32mmol (206mg) 9,9-dioctylfluorene-2,7-diboronate, 8.9mg catalyst Pd ( PPh 3 ) 4 , 2~3 drops of phase transfer catalyst CH 3 (C 8 h 17 ) 3 N + Cl -, into a 50ml round bottom flask. Connect the device and seal it, pass the inert gas Ar10min to remove the air in the device. Finally, 8ml of toluene and 1.9ml of sodium carbonate solution (2mol / L) were added, and gas was introduced for 5 minutes to remove the oxygen in the liquid to ensure that the reaction was carried out under the condition of cutting off the air. Start heating and stirring, and gradually raise the temperature to 90°C for reflux. After ref...
Embodiment 3
[0045] The preparation of 2,4,6-tribromo-1-octyloxybenzene and 9,9-dihexylbenzosilole hyperbranched polymer, the reaction formula is as follows:
[0046]
[0047] The preparation process of 2,4,6-tribromophenoxy n-octane is the same as in Example 1;
[0048] Take 0.2mmol (88mg) 2,4,6-tribromo-1-octyloxybenzene, 0.33mmol (199mg) 9,9-dihexylbenzosilole-2,7-diboronate, 9mg catalyst Pd (PPh 3 ) 4 , 2~3 drops of phase transfer catalyst CH 3 (C 8 h 17 ) 3 N + Cl - , into a 50ml round bottom flask. Connect the device and seal it, pass the inert gas Ar10min to remove the air in the device. Finally, 9ml of toluene and 2ml of sodium carbonate solution (2mol / L) were added, and gas was introduced for 5 minutes to remove the oxygen in the liquid, so as to ensure that the reaction was carried out under the condition of cutting off the air. Start heating and stirring, and gradually increase the temperature to 90°C for reflux. After reflux for 24 hours, inject a toluene solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com