Method for manufacturing lithium metal phosphate
一种锂金属磷酸盐、磷酸盐的技术,应用在非金属元素、碱金属化合物、化学仪器和方法等方向,能够解决粒子成长及工序费用增加等问题,达到工序便利、电池特性优异、特性提高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The present invention relates to the preparation method of lithium metal phosphate using crystalline iron phosphate or metal-doped crystalline iron phosphate as a precursor, and provides a lithium metal phosphate (LiMFePO) shown in the following formula I 4 , LMP) preparation method, comprising: the step of mixing slurry-like or cake-like crystalline iron phosphate and lithium raw material; and the step of heat-treating the above-mentioned mixture,
[0039] Formula I
[0040] Li M 1-n Fe n PO 4
[0041] Wherein, M is selected from the group consisting of Ni, Co, Mn, Cr, Zr, Nb, Cu, V, Ti, Zn, Al, Ga and Mg, and 0
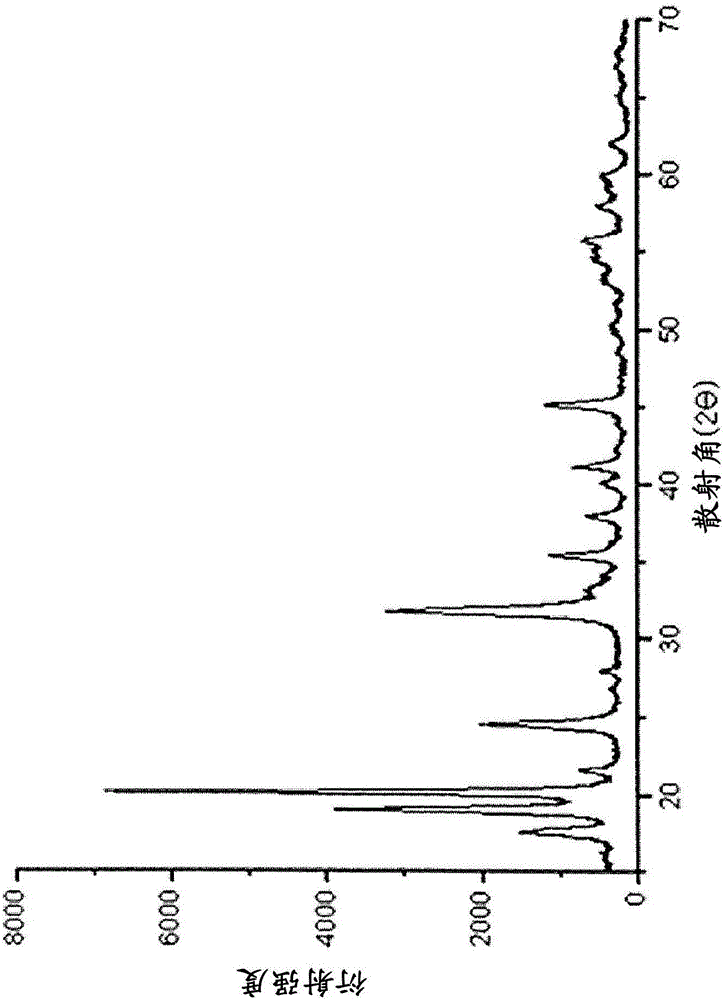
[0042] The crystalline iron phosphate (FP) is a (quasi) strengite structure in which octahedral and tetrahedral structures share corners (Corner-sharing), which is similar to the olivine structure of lithium iron phosphate (Olivine), that is, octahedral and tetrahedral structure sharing line-sharing (line-sharing) structure is similar, so compared w...
Embodiment 1
[0058] (1) Amorphous iron phosphate hydrate (amorphous FePO 4 2H 2 O) Synthesis
[0059] Weigh FeCl in such a way that the molar ratio of [Fe]:[P] is 1:1 3 ·6H 2 O and (NH 4 ) 2 HPO 4 and added to pure water for mixing to form a slurry. At this time, the ratio of the solid content to the solvent was 10%. Next, add ammonia water (NH 4 OH) to adjust the pH to 4.5. Next, the pH-adjusted slurry was stirred at 60° C. for 15 minutes. Next, the reaction slurry was washed three times with a centrifuge to obtain a cake-like non-crystalline iron phosphate hydrate.
[0060] (2) Crystalline iron phosphate hydrate (crystalline FePO 4 2H 2 O) Synthesis and Synthesis of LFP Using It
[0061] Pure water was added to the filter cake of amorphous iron phosphate hydrate obtained above to form a slurry. At this time, the volume ratio of the solid content to the solvent was 10%. Phosphoric acid (H 3 PO 4 ) to adjust the pH to 2. Next, the pH-adjusted slurry was stirred at 95°C fo...
Embodiment 2
[0063] (1) Amorphous iron phosphate hydrate (amorphous FePO 4 2H 2 O) Synthesis
[0064] In the same manner as in the above-mentioned Example 1, a filter cake-shaped amorphous iron phosphate hydrate was obtained.
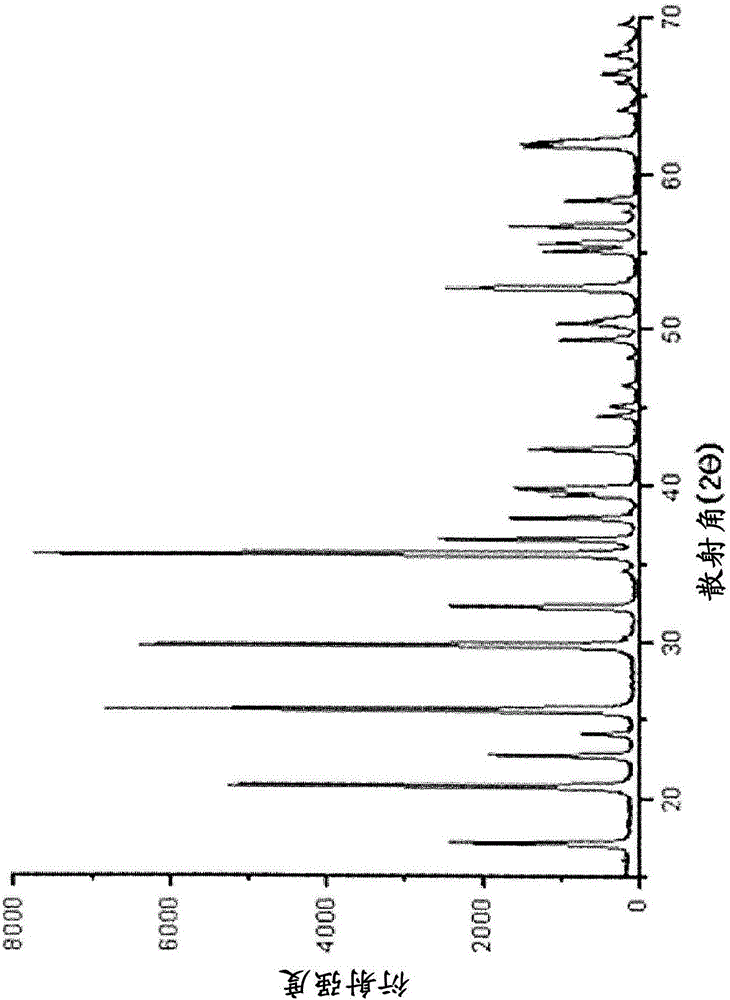
[0065] (2) Crystalline iron phosphate hydrate (crystalline FePO 4 2H 2 O) Synthesis and Synthesis of LFP Using It
[0066] Pure water was added to the non-crystalline iron phosphate hydrate filter cake obtained above to form a slurry. At this time, the volume ratio of the solid content to the solvent was 10%. Phosphoric acid (H 3 PO 4 ) to adjust the pH to 2. Next, the pH-adjusted slurry was stirred at 95° C. for 3 hours. At the point when the color of the slurry became light, the reaction was terminated. Then, the reaction slurry was washed 3 times with a centrifuge, and the water content of the washed filter cake was measured. On this basis, LiOH was added as a lithium raw material so that the molar ratio of [Li]:[Fe] was 1:1. Sucrose (sucrose) was adde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com