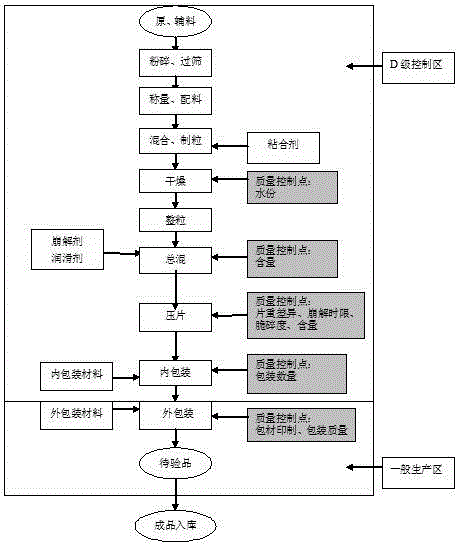
Production process for carbocisteine buccal tablet
A production process, the technology of carbocisteine, which is applied in the field of medicine, can solve problems such as troublesome taking, difficult to cover up the bad taste of drugs, etc., and achieve a one-sided and beautiful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A carbocisteine buccal unit production process, comprising the following steps
[0023] a. Crushing and sieving the raw and auxiliary materials, the fineness is 80 mesh of mannitol, 100 mesh of magnesium stearate, 60 mesh of menthol, 60 mesh of borneol, 80 mesh of PVPK, 100 mesh of sucrose, 100 mesh of protein sugar, and 100 mesh of carbocisteine;
[0024] b. Weighing and ingredients, carbocisteine 4.70 kg, mannitol 8.60 kg, sucrose 17.70 kg, color lake 0.007 kg, essence 0.011 kg, borneol 0.15 kg, take 0.20 kg of PVPK30 and 0.9 kg of purified water in the formula amount, stir after soaking Uniform, fast mixing for dry mixing;
[0025] c. Mix and granulate, add the prepared materials into the wet mixing granulator and dry mix for 3 minutes, add the prepared binder into the wet mixing granulator, wet mix quickly and wet mix for 1 minute, Add the wet-mixed material to a swinging granulator equipped with a 16-mesh nylon screen for granulation;
[0026] d. Drying, ...
Embodiment 2
[0033] A carbocisteine buccal unit production process, comprising the following steps
[0034] a. Crushing and sieving the raw and auxiliary materials, the fineness is 80 mesh of mannitol, 100 mesh of magnesium stearate, 60 mesh of menthol, 60 mesh of borneol, 80 mesh of PVPK, 100 mesh of sucrose, 100 mesh of protein sugar, and 100 mesh of carbocisteine;
[0035] b. Weighing and ingredients, carbocisteine 5.20 kg, mannitol 8.20 kg, sucrose 18.20 kg, color lake 0.002 kg, essence 0.016 kg, borneol 0.10 kg, take 0.15 kg of PVPK30 and 0.9 kg of purified water in the formula amount, stir after soaking Uniform, fast mixing for dry mixing;
[0036] c. Mix and granulate, add the prepared materials into the wet mixing granulator and dry mix for 3 minutes, add the prepared binder into the wet mixing granulator, wet mix quickly and wet mix for 1 minute, Add the wet-mixed material to a swinging granulator equipped with a 16-mesh nylon screen for granulation;
[0037] d. Drying, ...
Embodiment 3
[0044] A carbocisteine buccal unit production process, comprising the following steps
[0045] a. Crushing and sieving the raw and auxiliary materials, the fineness is 80 mesh of mannitol, 100 mesh of magnesium stearate, 60 mesh of menthol, 60 mesh of borneol, 80 mesh of PVPK, 100 mesh of sucrose, 100 mesh of protein sugar, and 100 mesh of carbocisteine;
[0046] b. Weighing and ingredients, carbocisteine 4.90 kg, mannitol 8.40 kg, sucrose 18.00 kg, color lake 0.004kg, essence 0.013 kg, borneol 0.13g, take 0.17 kg of PVPK30 and 0.9 kg of purified water in the formula amount, stir after soaking Uniform, fast mixing for dry mixing;
[0047] c. Mix and granulate, add the prepared materials into the wet mixing granulator and dry mix for 3 minutes, add the prepared binder into the wet mixing granulator, wet mix quickly and wet mix for 1 minute, Add the wet-mixed material to a swinging granulator equipped with a 16-mesh nylon screen for granulation;
[0048] d. Drying, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com