Use of manganite catalyst in catalytic oxidation of formaldehyde
A manganese oxide and catalytic oxidation technology, which is applied in the field of catalytic oxidation of formaldehyde and manganese oxide catalysts, can solve problems such as high temperature and cannot meet practical applications, achieve low ignition temperature, reduce harm to human health and ecological environment, The effect of simple and easy preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Prepare α, β, γ and δ crystal forms of manganese dioxide in the following manner:
[0031] With manganese sulfate as reducing agent, potassium permanganate or ammonium persulfate as oxidant, the manganese dioxide catalyst of α, β, γ and δ crystal forms is prepared by hydrothermal synthesis, as follows:
[0032] Add the required precursor (i.e. oxidizing agent and reducing agent) (X) and 80 mL deionized water to a 100 mL hydrothermal reaction kettle, thoroughly dissolve the precursor by stirring, and then place it in a constant temperature incubator with a certain reaction temperature (Y). React for a certain period of time (Z), and then filter, wash, and dry the contents of the reactor overnight, and then calcinate in air at 300°C. The following table lists the specific values for X, Y, Z.
[0033]
X
Y
Z
α-MnO 2
MnSO 4 ·H 2 O+KMnO 4
160℃
24h
β-MnO 2
MnSO 4 ·H 2 O+(NH 4 ) 2 S 2 O 8
140℃
1...
Embodiment 2
[0037] The α, β, γ and δ crystal forms of manganese dioxide were prepared in the same manner as in Example 1.
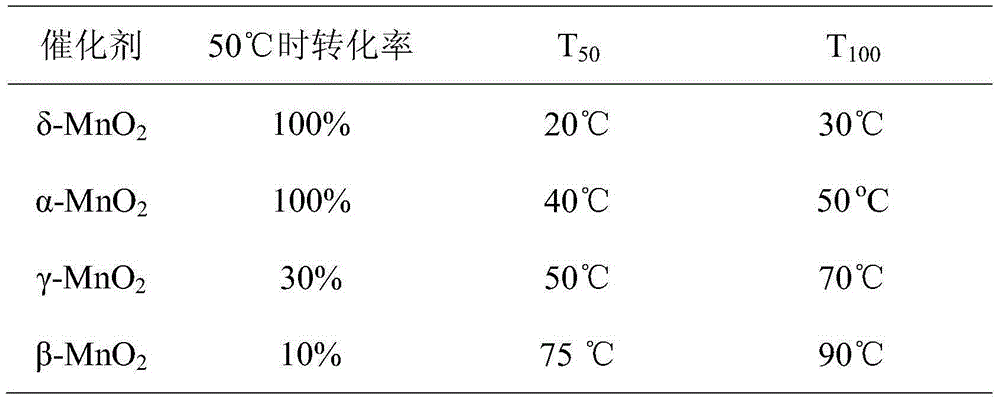
[0038] At a concentration of 170 ppm, a space velocity of 150,000 mL / (g h), and a temperature of 75 °C, the δ-crystalline manganese dioxide can maintain a 60% conversion rate for 50 h. α, β, γ are under the same conditions. Under the test, its activity gradually decreased after 5h.
Embodiment 3
[0040] The α, β, γ and δ crystal forms of manganese dioxide were prepared in the same manner as in Example 1.
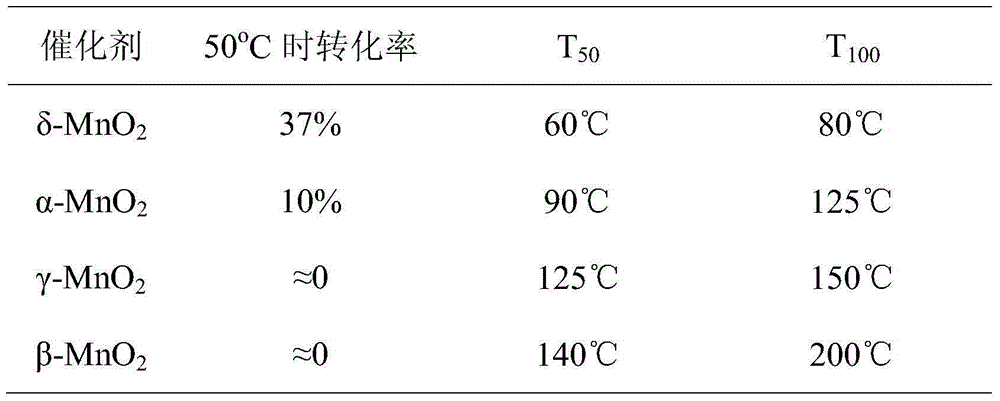
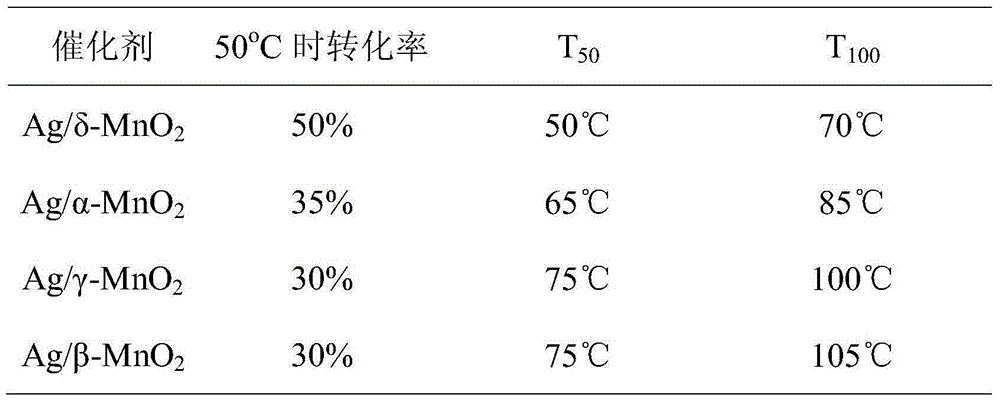
[0041] The relevant parameters of the catalytic formaldehyde activity of the manganese dioxide of the four crystal forms are listed in the following table, arranged in descending order of activity. Test conditions: concentration 5ppm, space velocity 5000mL / (g·h).
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com