MHCF/TiO2 nanocomposite catalyst as well as preparation method and application thereof
A nanocomposite, catalyst technology, applied in physical/chemical process catalysts, nanotechnology, nanotechnology and other directions, to achieve the effect of improving efficiency, good stability and recycling performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
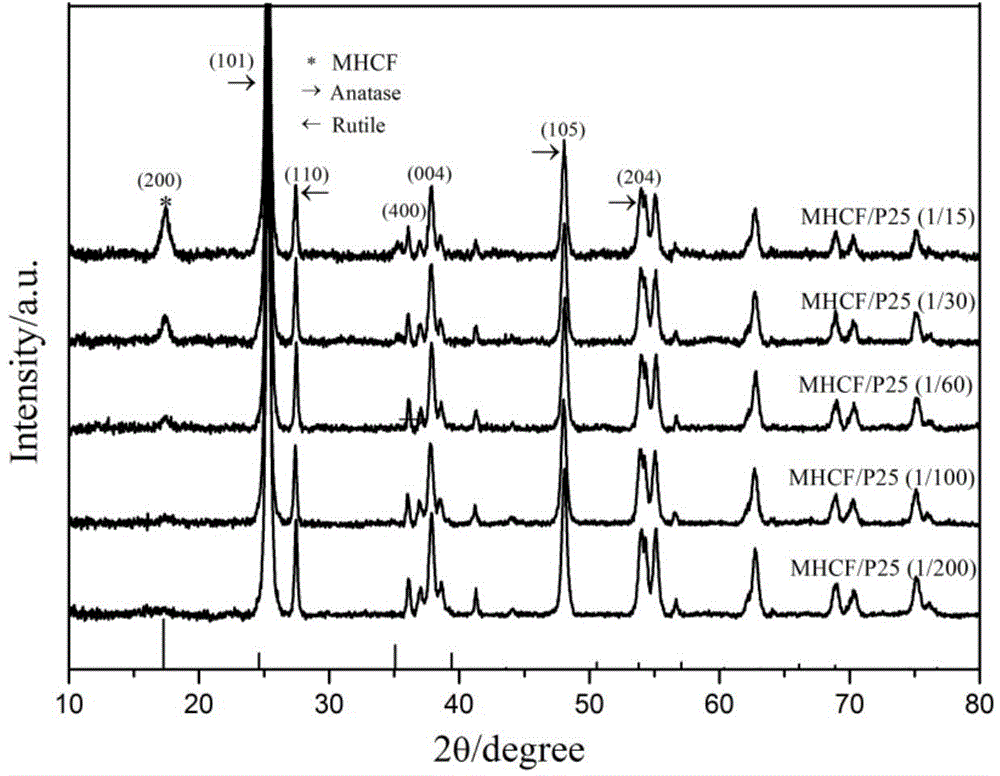
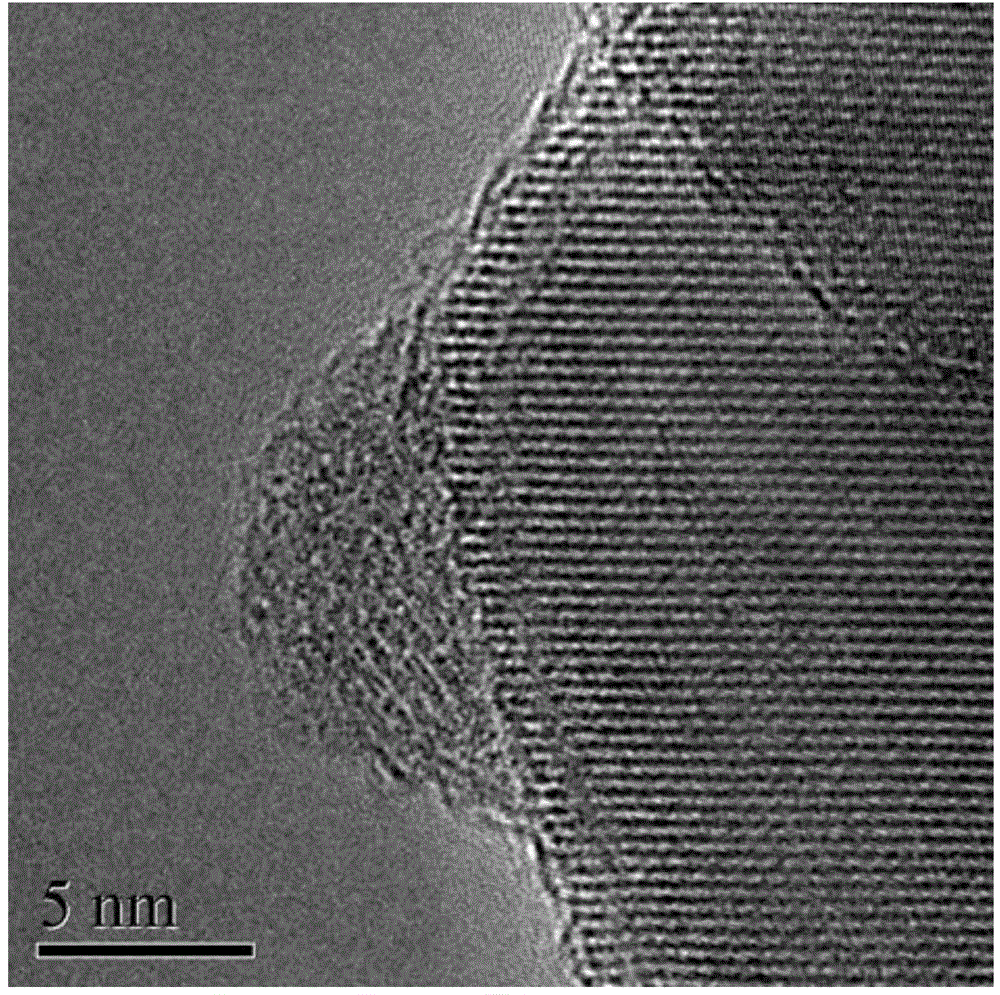
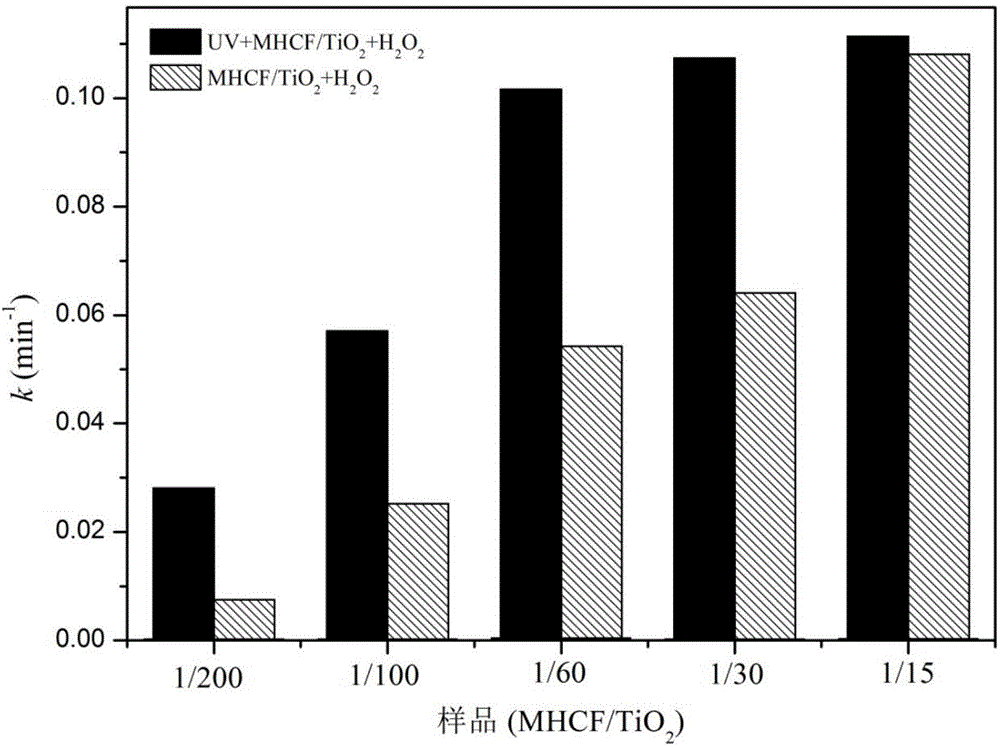
[0028] Disperse 0.95g of titanium dioxide (P25) in 15ml of deionized water and stir evenly, add 0.0254g of K 4 [Fe(CN) 6 ] Continue stirring for 30min to obtain solution a, dissolve 0.024g ferric chloride in 15ml deionized water to obtain solution b, slowly add solution b dropwise to a, continue stirring for 30min, and age for 20h. Prepare MHCF / TiO 2 =1 / 200 (molar ratio) nanocomposite material (observed by electron microscope, its particle size is 20-50 nanometers). Centrifugal washing and drying (drying at 20-80 degrees Celsius can be used) for light-Fenton synergistic catalytic activity test (dark reaction conditions: catalyst concentration 1g / L, RhB concentration 12mg / L, H 2 o 2 An aqueous solution with a concentration of 0.4mol / L. Photo-Fenton reaction conditions: add ultraviolet light on the basis of dark reaction: 27w black light lamp is 10cm height away from the liquid surface), after 30min, its activity of degrading RhB dark reaction is 22%, and the activity under ...
Embodiment 2
[0030] Disperse 0.95g of titanium dioxide (P25) in 15ml of deionized water and stir evenly, add 0.048g of K 4 [Fe(CN) 6 ]Continue stirring for 30min to obtain solution a, dissolve 0.0507g ferric chloride in 15ml deionized water to obtain solution b, slowly add solution b dropwise to a, continue stirring for 30min, and age for 20h. Prepare MHCF / TiO 2 =1 / 100 (molar ratio) of the nanocomposite material. After centrifugal washing and drying, it was used for light-Fenton synergistic catalytic activity test (dark reaction conditions: catalyst concentration 1g / L, RhB concentration 12mg / L, H 2 o 2 An aqueous solution with a concentration of 0.4mol / L. Photo-Fenton reaction conditions: add ultraviolet light on the basis of dark reaction: 27w black light lamp is 10cm away from the liquid surface). After 30min, its activity of degrading RhB dark reaction was 54%, and the activity under ultraviolet light irradiation was 83%.
Embodiment 3
[0032] Disperse 0.95g of titanium dioxide (P25) in 15ml of deionized water and stir evenly, add 0.0845g of K 4 [Fe(CN) 6 ]Continue stirring for 30min to obtain solution a, dissolve 0.0721g ferric chloride in 15ml deionized water to obtain solution b, slowly add solution b dropwise to a, continue stirring for 30min, and age for 20h. Prepare MHCF / TiO 2 =1 / 60 (molar ratio) of the nanocomposite material. After centrifugal washing and drying, it was used for light-Fenton synergistic catalytic activity test (dark reaction conditions: catalyst concentration 1g / L, RhB concentration 12mg / L, H 2 o 2 An aqueous solution with a concentration of 0.4mol / L. Photo-Fenton reaction conditions: add ultraviolet light on the basis of dark reaction: 27w black light lamp is 10cm away from the liquid surface). After 30 minutes, its activity of degrading RhB dark reaction was 81%, and the activity under ultraviolet light irradiation was 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com