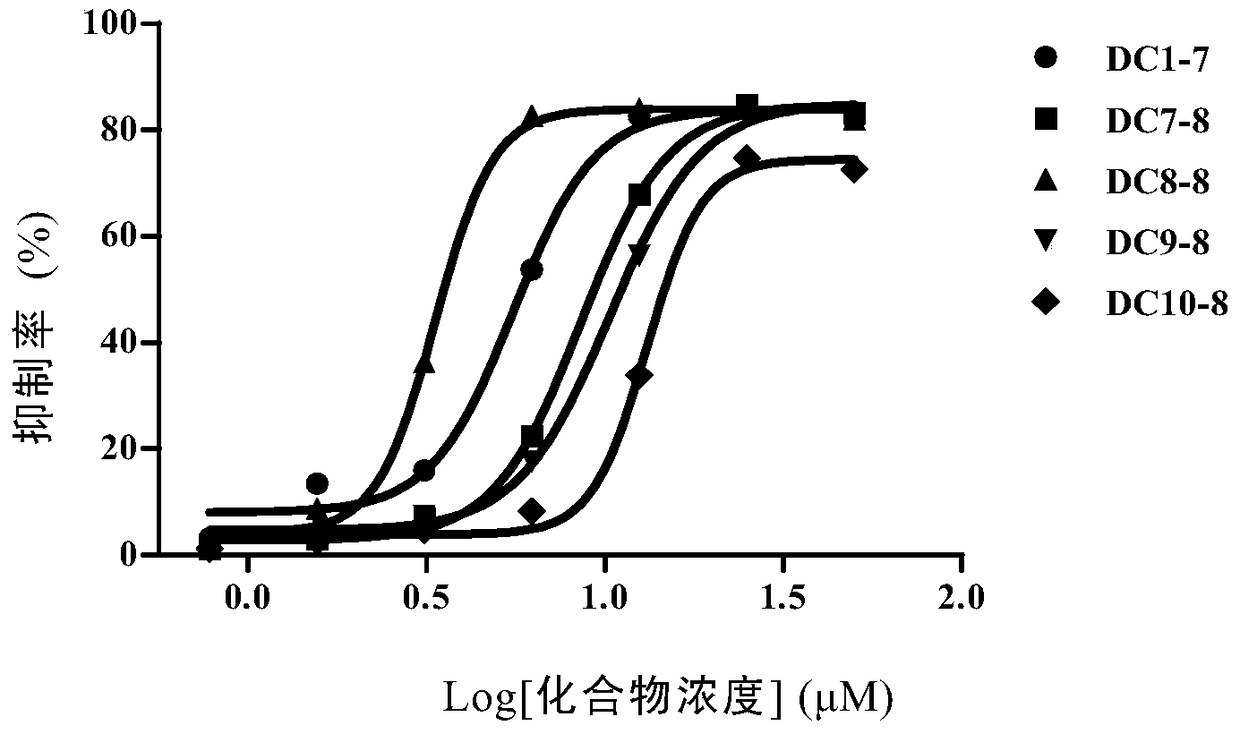
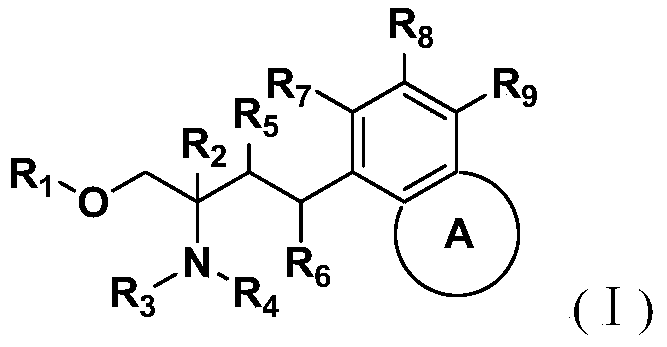
Amino alcohol compounds, their preparation methods, pharmaceutical compositions containing such compounds and their uses
A compound and composition technology, applied in the fields of medicinal chemistry and pharmacotherapy, can solve the problems of large side effects, short half-life, anorexia, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
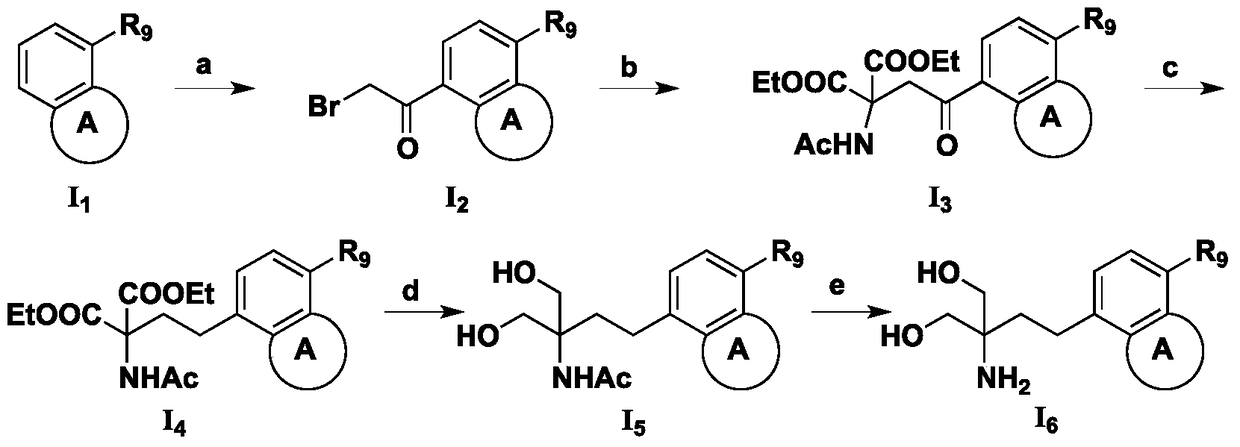
[0138] In a preferred example, the compound represented by the general formula I of the present invention is compound I 6 , the preparation method comprises the following steps:
[0139]
[0140] (a) Compound I 1 Reaction with bromoacetyl bromide gives compound I 2 ;
[0141] (b) Compound I obtained in step (a) 2 Reaction with diethyl acetamidomalonate to obtain compound I 3 ;
[0142] (c) Compound I obtained in step (b) 3 Compound I was obtained by reduction reaction 4 ;
[0143] (d) Compound I obtained in step (c) 4 Compound I is obtained through reduction reaction under the action of lithium aluminum hydride 5 ;
[0144] (e) Compound I obtained in step (d) 5 Compound I is obtained through reduction reaction under the action of lithium hydroxide 6 .
[0145] In another preferred example, the compound represented by the general formula I of the present invention is compound I 6 , the preparation method comprises the following steps:
[0146]
[0147] (a')...
Embodiment 1
[0243]
[0244] Step 1: Preparation of 1-n-octanoylnaphthalene
[0245] Add 6.38g (0.05mol) of naphthalene and 30mL of dichloromethane into a 100mL three-necked flask, cool down to -10°C, add 6.65g (0.05mol) of anhydrous aluminum trichloride in batches, and control the temperature below -10°C. A dichloromethane (5 mL) solution containing 8.1 g (0.05 mol) of n-octanoyl chloride was added dropwise, and reacted at this temperature for 10 h. The reaction solution was poured into ice water, extracted with ethyl acetate, the aqueous phase was extracted twice with ethyl acetate, the organic phases were combined, washed with saturated brine until neutral, dried over anhydrous magnesium sulfate, filtered and concentrated to obtain 9.2 g brown liquid. Petroleum ether was recrystallized to obtain 9.02 g of white crystals, with a yield of 71.2%.
[0246] 1 H NMR (400MHz, CDCl 3 )δ9.01(d,J=8.5Hz,1H),8.18-8.04(m,3H),7.68-7.50(m,3H),2.96(t,J=6.8Hz,2H),1.74-1.68(m ,2H),1.44-1.18(m,10H...
Embodiment 2
[0251]
[0252] Step 1: Preparation of 1-(2-bromoacetyl)-4-n-octylnaphthalene
[0253] Add 8.0g (33.3mmol) of 1-n-octylnaphthalene and 250mL of dichloromethane into a 500mL three-necked flask, and add 6.66g (49.9mmol) of anhydrous AlCl in batches at -20°C 3 A solution of 13.4 g (66.6 mmol) of bromoacetyl bromide in dichloromethane (150 mL) was added dropwise, and stirring was continued for 2 h. Pour the reaction solution into 500g of ice under vigorous stirring, bring it up to room temperature, separate the dichloromethane layer, extract the aqueous phase with dichloromethane (100mL×2), combine the organic phases, and wash with saturated brine until neutral (100mL×2 ), dried over anhydrous magnesium sulfate, filtered and concentrated, and recrystallized with 60mL of methanol to obtain 9.5g of light yellow crystals with a yield of 79.8%, which was directly used in the next step.
[0254] Step 2: Preparation of Diethyl 2-Acetamido-2-(2-(4-octylnaphthalen-1-yl)-2-carbonylethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com