Cyanine compound and application thereof
A compound and cyanine technology applied in the field of fluorescent probes to improve detection accuracy and reduce interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1. Preparation of cyanine compound:
specific Embodiment
[0051] The cyanine-based fluorophore shown in the general formula I comes from commercially available products, and then different positioning groups are respectively modified on the corresponding positions of the fluorophore. Finally, the corresponding cyanine compound is obtained by reacting the fluorophore modifying the positioning group and the substituted benzoic acid in dichloromethane solvent. Specific examples are as follows:
[0052] Preparation of a compound of formula:
[0053] Under argon protection, 2,2'-dithiodibenzoic acid (1.53g, 5mmol) and oxalyl chloride (2.96g, 23.3mmol) were reacted in 100ml of dichloromethane for 3 hours in an ice-water bath, and the solvent was evaporated to dryness stand-by. Under the ice-water bath, the cyanine fluorophore (500mg, 1.08mmol) was dissolved in dichloromethane, then 2,2'-dithiodibenzoyl chloride was added dropwise, reacted under the ice bath for 1 hour, and then washed with water until neutral. Then dichloromethane (100m...
Embodiment 2
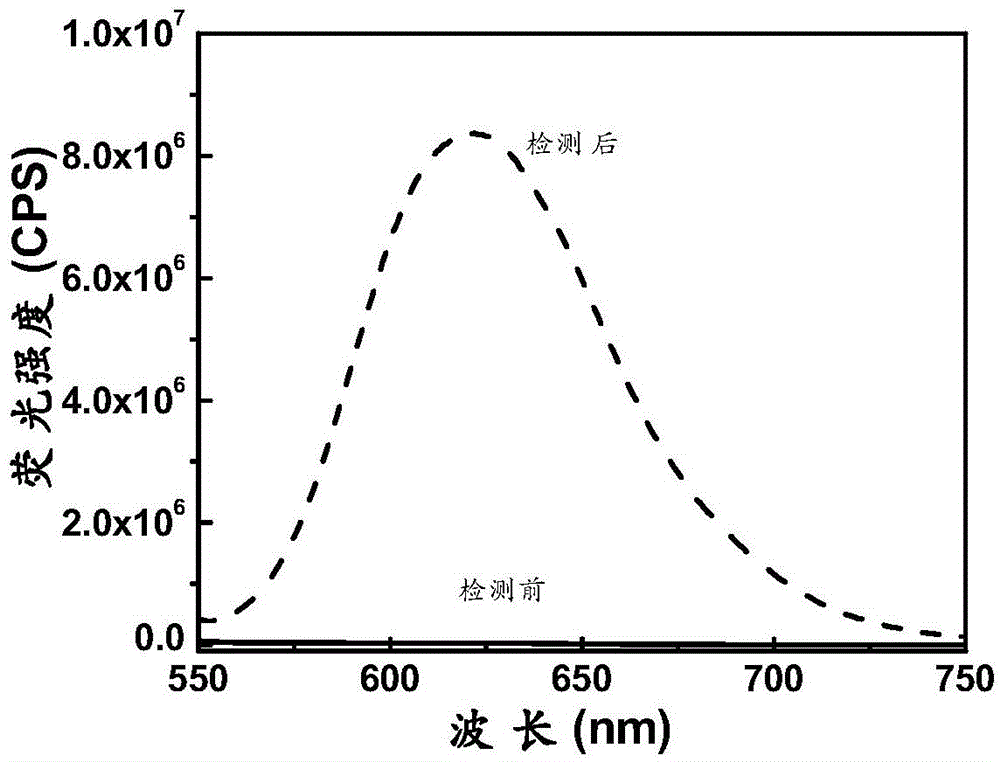
[0067] The prepared compound of formula one is used as a probe in the water system, simulated physiological environment and intracellular detection of sulfane sulfur, simulated physiological conditions, the following experiments are all carried out under the condition of pH=7.4 (HEPES buffer solution, concentration 40 mM), the probe concentration was 2 μM.
[0068] The response of the above-mentioned prepared compound formula to superoxide anion:
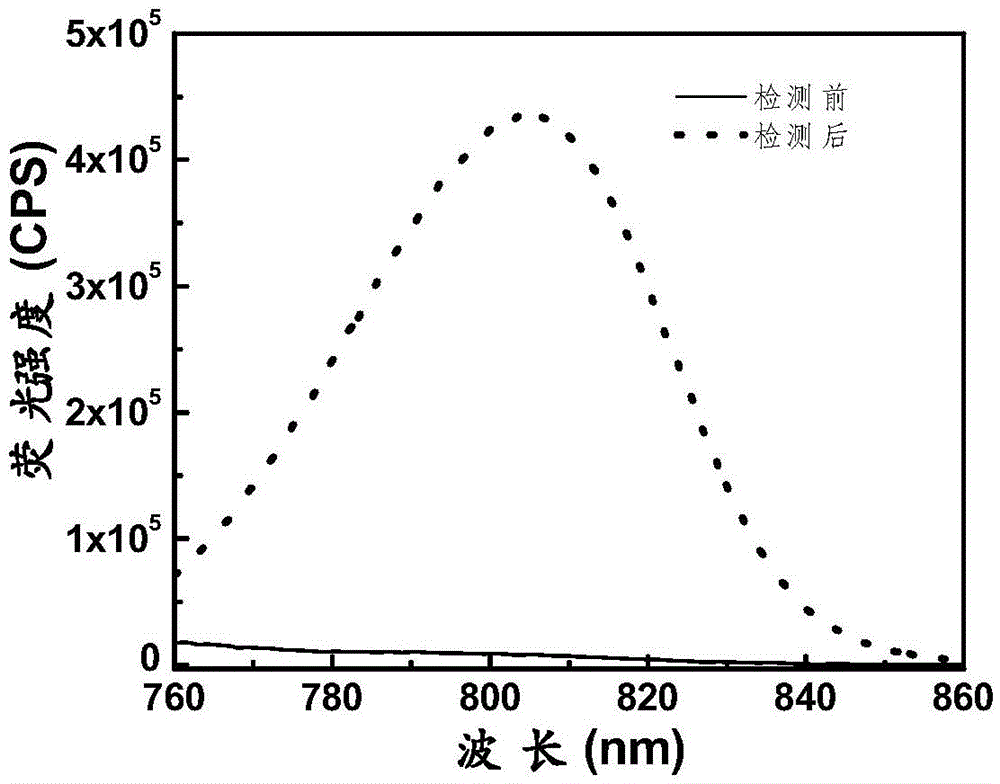
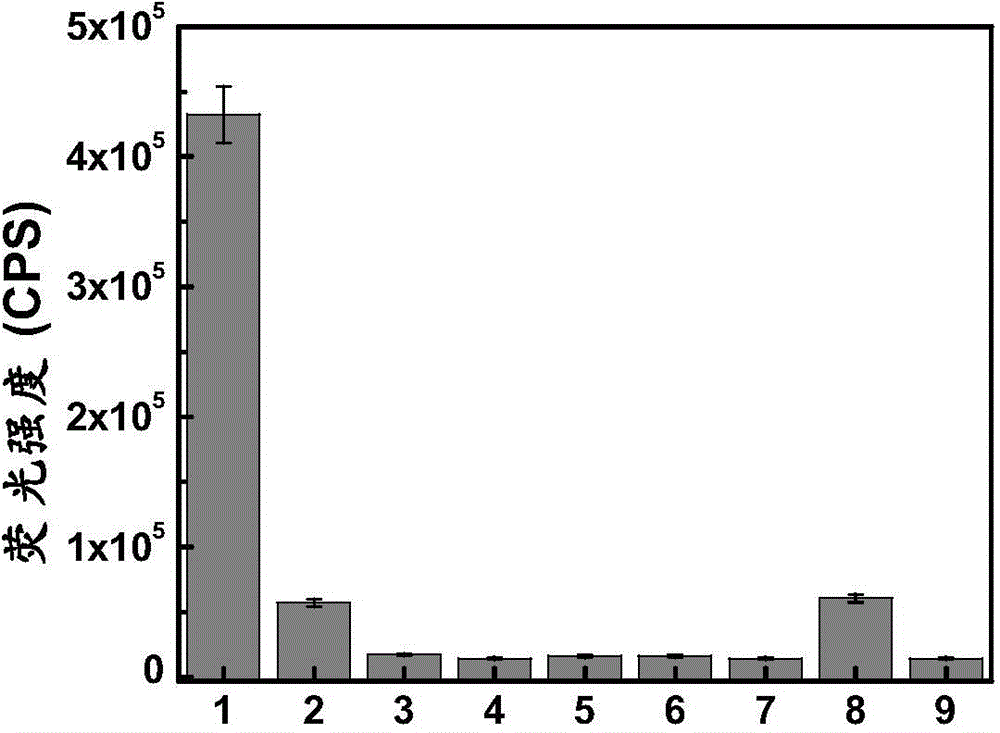
[0069] pH was controlled with HEPES buffer solution. Add 2μM compound formula 1 to a 10ml colorimetric tube, then add 40mM HEPES, then add 5μM superoxide anion, dilute the volume to 10ml with ultrapure water, shake the solution, and equilibrate for 10min, then add the above working solution into a fluorescent dish to measure the fluorescence spectrum. The changes of the fluorescence spectrum before and after the detection of superoxide anion are as follows: figure 1 shown. The compound can be used to realize the detection of sup...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com