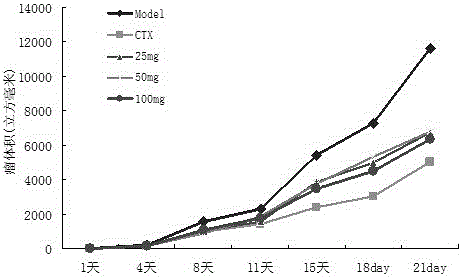
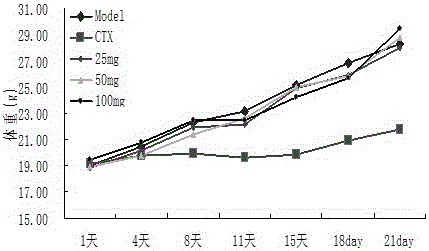
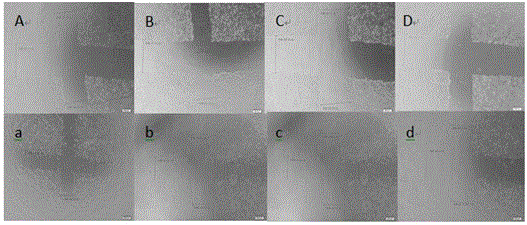
Divaricate velvetplant root and rhizome polysaccharide and application thereof in preparing medicine and functional food for immune adjustment and tumor resistance
A technology of Panax notoginseng and functional food, applied in the field of medicine, can solve the problems of immune regulation and antitumor activity of Panax notoginseng polysaccharide, which have not been reported, and achieve good market application prospects, improve the level of white blood cells, and inhibit migration. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1, Extraction, Preparation and Analysis of Panax notoginseng Polysaccharide
[0026] The preparation method of Panax notoginseng polysaccharide is as follows: take 50 g of Panax notoginseng stems and leaves, dry and pulverize them, pass through a 50-mesh sieve to obtain Panax notoginseng powder, add 20 times the mass of deionized water, and extract by reflux at 70-100°C for 2 hours. Centrifuge, take the supernatant, and add 5 times the mass of deionized water to the precipitate, reflux at 70-100°C for 2 hours, centrifuge, and combine the filtrate; add a certain amount of 95% ethanol to the combined filtrate to make the final ethanol concentration 75%, alcohol precipitation overnight, centrifugation to remove the supernatant, the precipitate is deproteinized and decolorized, and then freeze-dried, and the finally obtained biologically active polysaccharide is the Panax notoginseng polysaccharide of the present invention.
[0027] According to HPLC molecular weigh...
Embodiment 2
[0028] Example 2, the promoting effect of Panax notoginseng polysaccharide on the phagocytosis of neutral red in RAW264.7 cells
[0029] Mouse mononuclear macrophage RAW264.7 cells were cultured in DMEM high glucose medium containing 10% fetal bovine serum. Cells in the logarithmic growth phase were taken at 1.0×10 5 The concentration of cells / mL was inoculated in a 96-well plate in 5% CO 2 , 37°C, cultured in a constant temperature incubator with saturated humidity until the cells adhered to the wall (about 6-8h), adding different concentrations of Panax notoginseng polysaccharides (1, 5, 10, 25, 50, 100 μg / ml) for a total of 6 concentrations, the positive drug is lentinan, the final concentration is 2 μg / ml, set 4 parallel wells for each concentration, take it out after 12 hours of culture, absorb the original medium, wash it once with PBS at room temperature, add 100 μL of 0.075% medium to each well Neutral red staining solution, continue to incubate for 30 minutes, absor...
Embodiment 3
[0034] Example 3, the effect of Panax notoginseng polysaccharide on promoting the secretion of nitric oxide from RAW264.7 cells
[0035] Mouse mononuclear macrophage RAW264.7 cells were cultured in DMEM high glucose medium containing 10% fetal bovine serum. Cells in the logarithmic growth phase were inoculated in 96-well plates at a concentration of 1.0x105 cells / mL, cultured in a constant temperature incubator with 5% CO2, 37°C, and saturated humidity until the cells adhered to the wall (about 6-8 hours), and then added There are 6 concentrations of different concentrations of Panax notoginseng polysaccharide (1, 5, 10, 25, 50, 100 μg / ml), the positive drug is lentinan, the final concentration is 2 μg / ml, and 4 parallel wells are set for each concentration. Take it out after 24 hours of cultivation, take 50 μL of the culture base into another 96-well plate, and detect it according to the instruction of the nitric oxide detection kit (Beiyuntian Bioreagent Company), that is, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com