Synthesis methods of methyl esters of 1-amino-cycloheptane-carboxylic acid
A technology for methyl phenylcycloheptanecarboxylate and phenylcycloheptanecarboxylic acid, which is applied in the field of synthesis of 1-phenylcycloheptanecarboxylic acid and its esters, and can solve the problem of cumbersome operations, long and shortened synthetic routes Reaction routes and other issues to achieve the effects of simplifying reaction operations, shortening reaction steps, saving energy and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
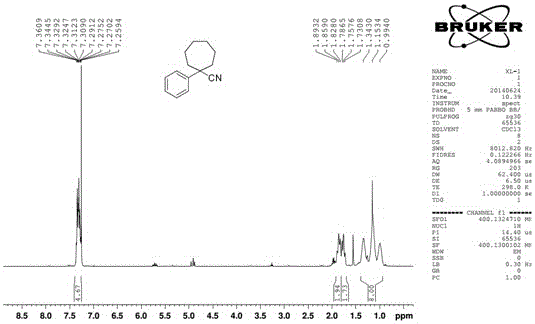
[0028] Add 47.86g (0.30mol) of t-BuOK to a 500mL three-necked flask, a small amount of KI, stir with 100mL of DMF, control the temperature in an ice-salt bath below 0°C, and dropwise add [10.00g (0.085mol) of phenylacetonitrile plus DMF50mL] mixed solution, Keep the temperature below 0°C, drop it for 30 minutes, stir for 30 minutes, add 20.57g (0.085mol) of 1,6-dibromohexane in 50mL DMF mixed solution dropwise, keep the temperature below 0°C, raise the temperature to 80°C, and react for 4h Afterwards, TLC detected that the reaction was complete, the reaction solution was poured into cold water, extracted 3 times with EA, the organic layers were combined, washed once with water, washed once with saturated NaCl solution, and washed once with anhydrous NaCl solution. 2 SO 4 Dry, filter and concentrate the next day. 1 H-NMR (400MHz, CDCl 3 )δ: 7.36~7.26(m,5H), 1.89~1.83(m,2H), 1.79~1.73(m,2H), 1.34~0.99(m,8H).
[0029] Add 85.6 mL of ethylene glycol and 24.08 g (0.43 mol) of KO...
Embodiment 2
[0032] Add t-BuOK33.60g (0.30mol) and a small amount of KI to a 500mL three-necked flask, stir in 100mL of DMF, control the temperature in an ice-salt bath below 0°C, add dropwise a 50mL DMF solution of 8.99g (0.06mol) of methyl phenylacetate, and keep When the temperature is below 0°C, after 30 minutes of dripping, after stirring for 30 minutes, add 1,6-dibromohexane 13.50g (0.06mol) in 50mL of DMF dropwise, keep the temperature below 0°C, raise the temperature to 80°C, and react for 4 hours, TLC detected that the reaction was complete, the reaction solution was poured into cold water, extracted 3 times with EA, combined the organic layers, washed once with water, washed once with saturated NaCl solution, and washed once with anhydrous NaCl solution. 2 SO 4 Dry, filter and concentrate. Purification by column chromatography was eluted with petroleum ether to obtain the product. Yield 88.6%, HPLC purity 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com