Doxofylline compound and medicine composition thereof
A technology of doxofylline and compound, applied in the field of medicine, can solve the problems of reduced active ingredient content, insufficient quality stability, unfavorable clinical drug safety and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
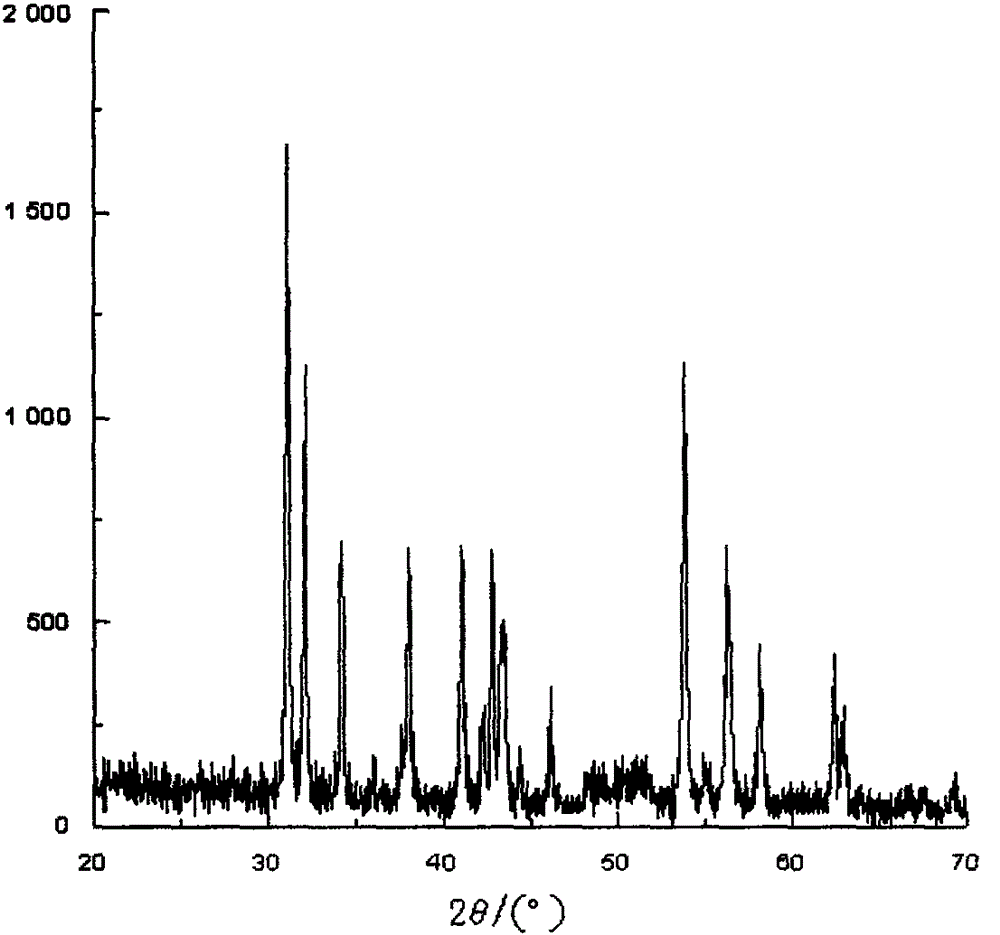
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 doxofylline compound
[0038] At 50-60° C. and a stirring speed of 100-150 rpm, theophylline and 2-bromo-1,3-dioxolane are added to the reactor in a weight ratio of 1:1.2. After adding sodium carbonate as a catalyst and dimethylformamide as a solvent, the temperature starts to rise, and the reaction temperature is kept at 110-115°C. After the reaction was finished, dimethylformamide was recovered by distillation under reduced pressure. Cool the reaction to 20-25°C, filter and rinse with pure water. To the filtered crude product of doxofylline, add about 1.5% of the weight of doxofylline with activated carbon and a small amount of toluene, stir at 150-200 rpm for 30 minutes, filter to remove carbon, and filter the filtrate through a 0.22 μm filter membrane. The toluene was then removed by drying at 95-100°C for 6 hours. The doxofylline is dried to a constant weight in a vacuum tray dryer at 95-100° C., namely the doxofylline dry powder....
Embodiment 2
[0039] The preparation of embodiment 2 doxofylline compound
[0040] At 50-60° C. and a stirring speed of 100-150 rpm, theophylline and 2-bromo-1,3-dioxolane are added to the reactor in a weight ratio of 1:1. After adding sodium carbonate as a catalyst and dimethylformamide as a solvent, the temperature starts to rise, and the reaction temperature is kept at 110-115°C. After the reaction was finished, dimethylformamide was recovered by distillation under reduced pressure. Cool the reaction to 20-25°C, filter and rinse with pure water. To the filtered crude product of doxofylline, add about 1.5% of the weight of doxofylline with activated carbon and a small amount of toluene, stir at 150-200 rpm for 30 minutes, filter to remove carbon, and filter the filtrate through a 0.22 μm filter membrane. The toluene was then removed by drying at 95-100°C for 6 hours. The doxofylline is dried to a constant weight in a vacuum tray dryer at 95-100° C., namely the doxofylline dry powder. ...
Embodiment 3
[0041] The preparation of embodiment 3 doxofylline injection
[0042] prescription:
[0043] Doxofylline Compound 10g
[0044] Water for injection 1L
[0045] Made of 100
[0046]Dissolve the prescribed amount of doxofylline compound with 80% of the total amount of water for injection at 50°C, adjust the pH to 5.8-6.4 with hydrochloric acid, add water for injection to the full amount, stir evenly, filter with a 0.22 μm filter membrane, and fill in an ampoule , filled with nitrogen, melt-sealed, and sterilized with damp heat at 121° C. for 30 minutes to obtain 10 g: 10 ml doxofylline injection.
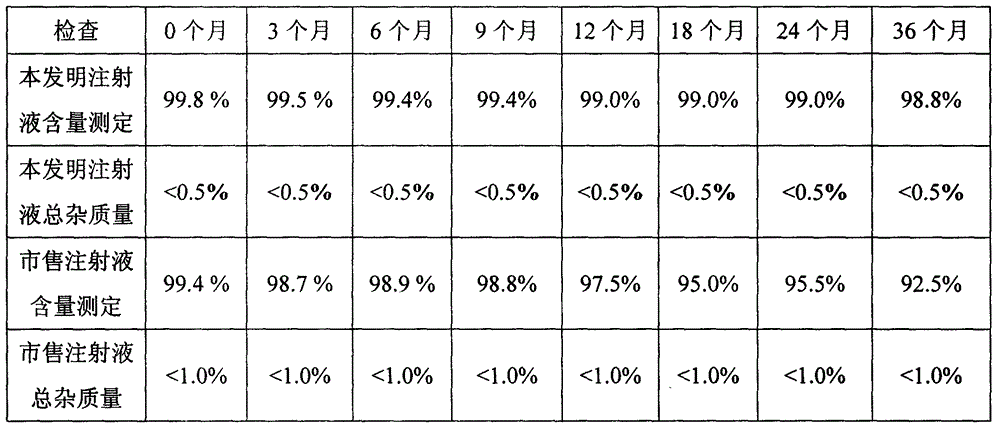
[0047] The doxofylline injection prepared in Example 3 of this experiment and the commercially available doxofylline injection were investigated for long-term stability (25°C±2°C; 60%±5%RH), and the results are shown in Table 1.
[0048] Table 1 Long-term stability investigation table of doxofylline injection
[0049]
[0050] Conclusion: The quality indicators of doxofylline i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com