Effective rare earth complex luminescent material excited by shortwave ultraviolet
A rare earth complex and rare earth technology, applied in luminescent materials, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the complex structure of the complex, the material luminous efficiency has not been greatly improved, and rare earth ions cannot be obtained Saturation coordination and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1. The synthetic route involved in the present embodiment is as follows:
[0028]
[0029] (1) Synthesis of DPPOPyC (2-carboxy-6-diphenylphosphoryloxypyridine, 6-diphenylphosphorylpicolinic acid)
[0030] Add 10mmol of diphenylphosphine chloride to a tetrahydrofuran solution of metal sodium (20mmol), heat to reflux for 12h, add 10mmol of the sodium salt of 2-carboxylic acid-6-bromopyridine to freshly prepared sodium diphenylphosphine (( Ph) 2 PNa) solution, heated to reflux for 12h. After the reaction was completed, an equivalent amount of m-chloroperoxybenzoic acid (mCPBA) was added, stirred for 1 h, and then the pH of the solution was adjusted to 2-3 with 2M HCl. After column separation, methanol recrystallization, and vacuum drying, 1.8 g of white powder can be obtained with a yield of 56%.
[0031] 1 HNMR (400MHz, CDCl 3): δ8.49(ddd, J=7.5, 5.4, 0.9Hz, 1H), 8.33(d, J=7.9Hz, 1H), 8.14(td, J=7.8, 3.2Hz, 1H), 7.90-7.73( m, 4H), 7.59 (td, J=7.3, 1.2 ...
Embodiment 2
[0035] Example 2. Synthesis of the rare earth complex shown in formula IV-2 (i.e. R=DPPO in formula IV, i.e. diphenylphosphineoxy, Ln=Eu)
[0036]
[0037] The synthesis steps are the same as in Example 1, except that the rare earth salt is replaced by europium trichloride hexahydrate. 0.95 g of white powder of the target europium complex was obtained. Mass spectrometry (m / z, ESI): Calc. 1119.1, found 1120.1 (M+H). Elemental analysis (mass percentage): C, 57.20 (57.19); H, 3.70 (3.62); N, 3.76 (3.71), Eu(DPPOPyC)3·0.9H in brackets 2 O theoretical value.
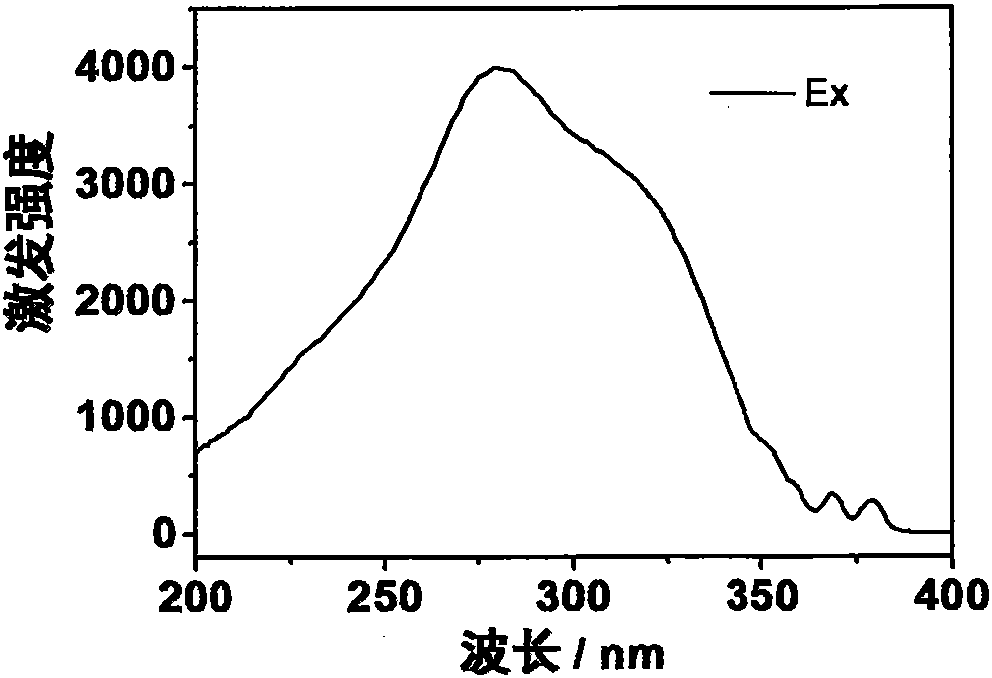
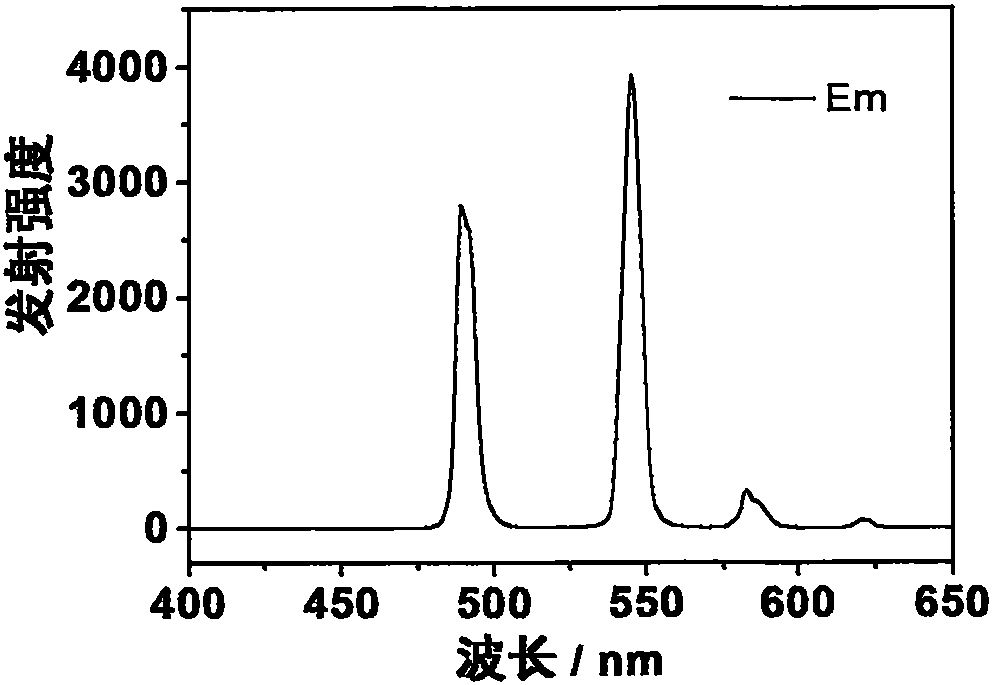
[0038] The prepared europium complex Eu(DPPOPyC)3 can obtain bright red emission under the excitation of ultraviolet lamp. The emission spectrum (excitation wavelength is 280nm) of its solid powder is as image 3 shown. The quantum yield of solid powder is as high as 93%.
Embodiment 3
[0039] Example 3. Synthesis of the rare earth complex shown in formula IV-3 (i.e. R=DPPO in formula IV, i.e. diphenylphosphineoxy, Ln=Dy)
[0040]
[0041] The synthesis steps are the same as in Example 1, except that the rare earth salt is replaced by dysprosium trichloride hexahydrate. 1.0 g of white powder of the target dysprosium complex was obtained. Mass spectrometry (m / z, ESI): Calc. 1130.1, found 1131.1 (M+H). Elemental analysis (mass percentage): C, 53.30 (53.22); H, 4.17 (4.19); N, 3.40 (3.39), Dy(DPPOPyC)3 0.9CH in brackets 3 OH·4.5H 2 O theoretical value.
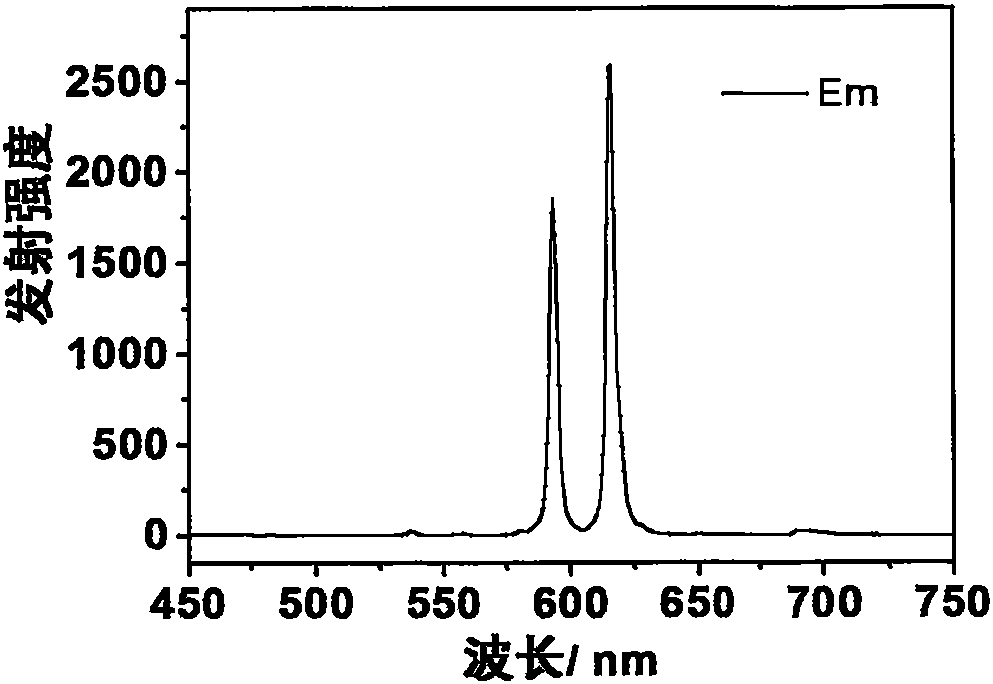
[0042] Dysprosium complex Dy(DPPOPyC) prepared 3 Yellow-white light emission can be obtained under the excitation of ultraviolet lamp. Emission spectrum (excitation wavelength is 28lnm) such as Figure 4 shown. The quantum yield of solid powder can reach 10%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com