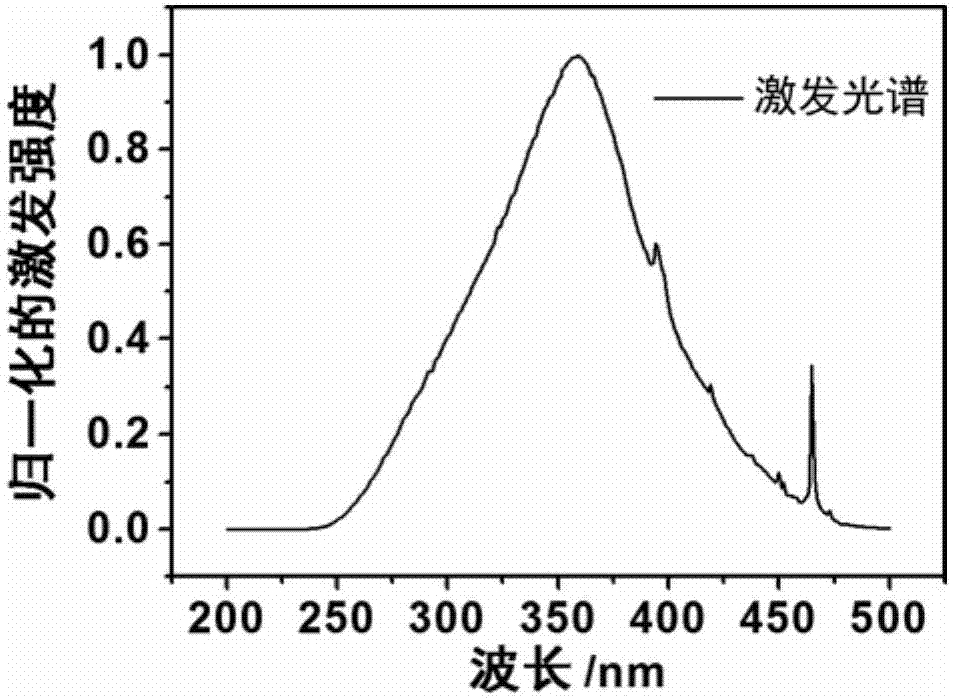
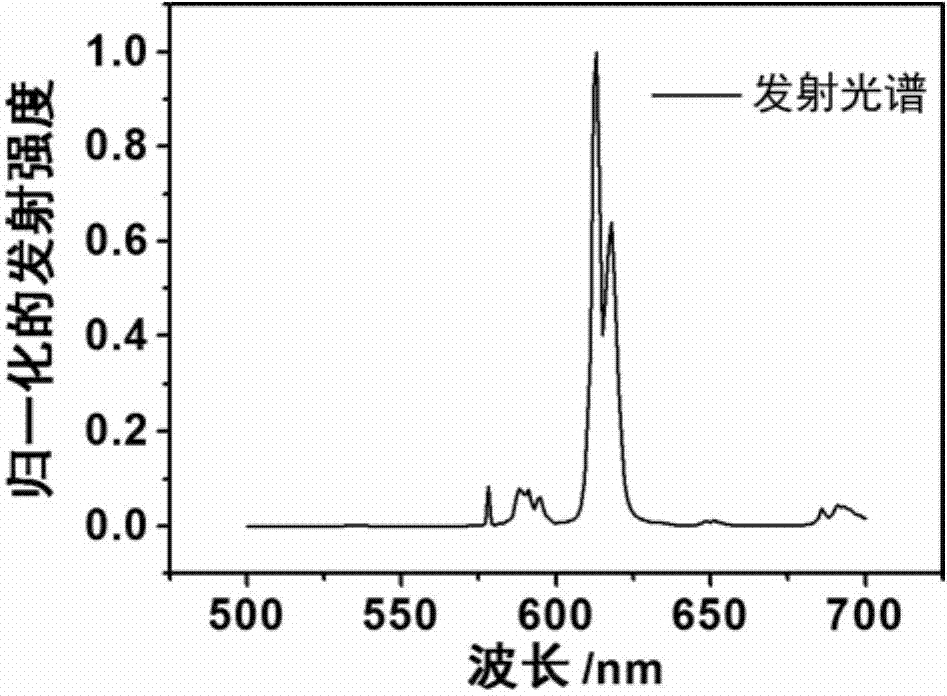
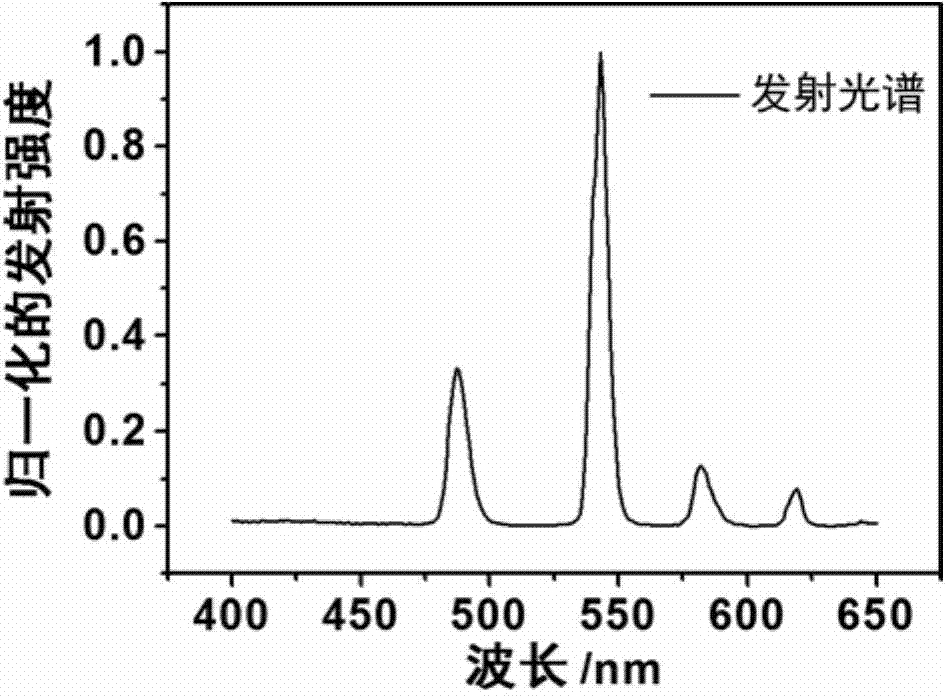
Ionic rare-earth complex luminescent material and preparation method and application therefor
A technology of rare earth complexes and ions, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of poor photostability, poor stability, and easy dissociation of neutral ligands, etc., and achieve stable complex structures, Excludes quenching, strong interaction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1. the rare earth complex compound shown in synthetic formula II-1 (being R in the formula II 1 =CN,R 3 =CH 3 , R 2 = R 4 = R 5 =H, Ln=Eu, M=Na)
[0040]
[0041] With 4mmol of 8mCND (i.e. R in formula I 1 =CN,R 3 =CH 3 , R 2 = R 4 = R 5 =H) and 4 mmol of NaOH in a mixed solution of ethanol and water 1:1 (volume ratio) were heated to reflux for 30 minutes. Then, 1 mmol of an aqueous solution of europium trichloride hexahydrate was added dropwise to the above solution or suspension, and refluxed for 2 hours. Filtered, washed with water, washed with a small amount of ethanol, and dried in vacuum. After recrystallization from ethanol / chlorobenzene, 0.81 g of the white target europium complex was obtained.
[0042] Mass spectrometry (ESI-MS) analysis, measured molecular ion peak M / Z=889.1, [M-Na] - . Elemental analysis (mass percentage): C, 52.70 (52.88); H, 2.65 (2.67); N, 18.44 (18.51), the theoretical values in brackets. Dissolving the com...
Embodiment 2
[0043] Embodiment 2. the rare earth complex shown in synthetic formula II-2 (being R in the formula II 1 = R 3 =CH 3 , R 2 = R 4 = R 5 =H, Ln=Eu, M=Na)
[0044]
[0045] The synthesis steps are the same as in Example 1, except that the ligand is changed to 2m8mND (i.e. R in formula I 1 = R 3 =CH 3 , R 2 = R 4 = R 5 = H). 0.69 g of the light yellow target europium complex was obtained. Mass spectrometry (ESI-MS) analysis, recorded molecular ion peak M / Z=845.2, [M-Na] - . Dissolving the complex represented by formula II-2 in acetone solution, or in the form of solid powder, can obtain bright red emission under the excitation of ultraviolet lamp.
Embodiment 3
[0046] Embodiment 3. the rare earth complex shown in synthetic formula II-3 (being R in the formula II 1 = CF 3 , R 3 =CH 3 , R 2 = R 4 = R 5 =H, Ln=Eu, M=Na)
[0047]
[0048] The synthesis steps are the same as in Example 1, except that the ligand is changed to 3CF 3 8mND (that is, R in formula I 1 = CF 3 , R 3 =CH 3 , R 2 = R 4 = R 5 = H). 0.88 g of white powder of the target europium complex was obtained. Mass spectrometry (ESI-MS) analysis, recorded molecular ion peak M / Z=1061.1, [M-Na] - . Dissolving the complex represented by formula II-3 in acetone solution, or in the form of solid powder, can obtain bright red emission under the excitation of ultraviolet lamp.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com