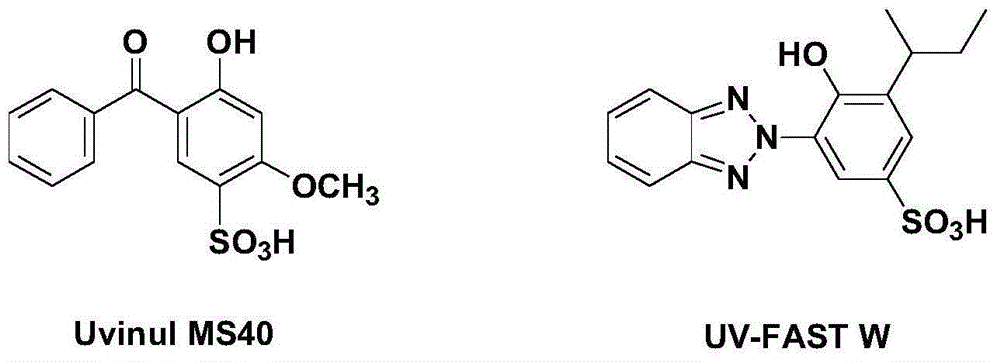
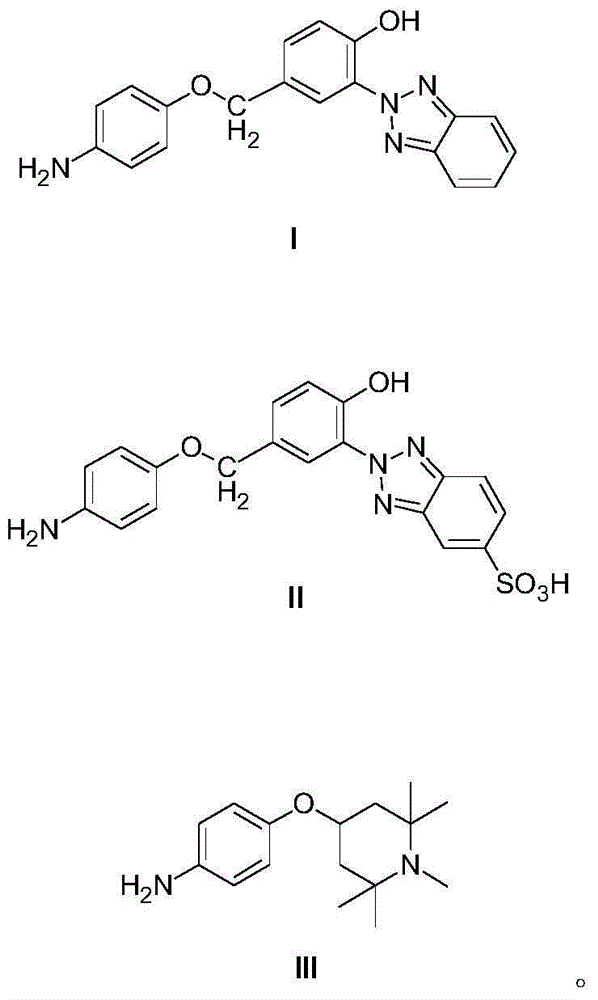
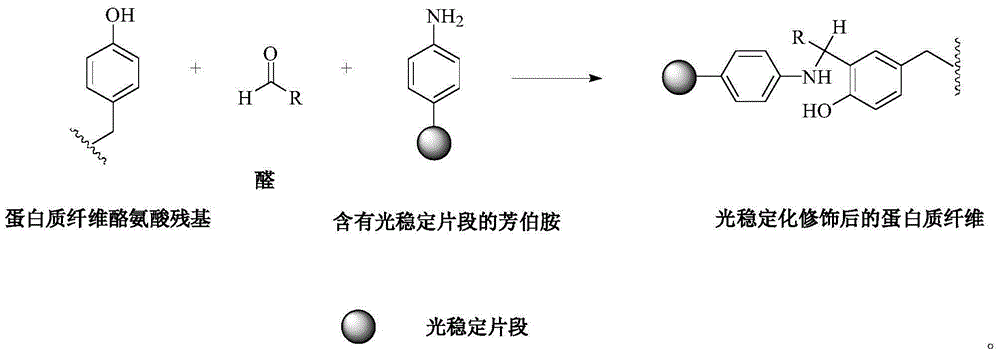
Reactive light stabilizer suitable for protein fibers and preparing method thereof
A protein fiber, light stabilizer technology, applied in fiber processing, animal fibers, textiles and papermaking, etc., can solve the problems of poor wet durability and limited application, achieve low development cost, low energy consumption, and solve the problem of wet effect on state durability and compatibility issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation method of , the reaction formula is as follows:
[0036]
[0037] Follow these steps in sequence:
[0038] 1), respectively take 0.75g (0.003mol) UV-P (2-(2'-hydroxy-5'-methylphenyl) benzotriazole), 0.02g azobisisobutyronitrile (AIBN), 30mL of tetrachloromethane was added to a 250mL three-necked flask, stirred to dissolve completely, and heated to 47°C; 0.53g (0.003mol) of bromine was added to a beaker containing 20mL of tetrachloromethane, and shaken well and controlled at 4~ It was added to the above three-necked flask within 5h (the temperature of the system in the three-necked flask was kept at 47°C), and the reaction was carried out at 47°C for 17h. After the reaction, the temperature was raised, and the product was distilled under reduced pressure (at a pressure of 9.9 mmHg, the fraction at 80° C. was collected) to obtain the product monobrominated UV-P.
[0039] 2) Dissolve 0.6g (0.015mol) NaOH in 30mL deionized water, transfer it to a thre...
Embodiment 2
[0042]
[0043] Using UV-P sulfonic acid as the starting material to replace UV-P, the molar weight remains unchanged; other reaction steps are identical to those in Example 2, and the target product II is obtained.
[0044] Remarks: Step 1) under reduced pressure distillation to a pressure of 9.9 mmHg, after evaporating the solvent to dryness, the remaining substance is the monobrominated product.
[0045] In step 2), atmospheric distillation is used to remove the solvent, and the remaining substance is the intermediate nitro light stabilizer II.
[0046] See reference for the preparation method of UV-P sulfonic acid: PhotoprotectionofWoolwithSulfonated2-(2'-Hydroxyaryl)-2H-benzotriazoles.PolymerDegradationandStability14(1986)263-284.
Embodiment 3
[0048]
[0049]
[0050] Follow these steps in sequence:
[0051] 1), add 17.4mL (0.24mol) of thionyl chloride to the 100mL three-necked flask, under stirring conditions, add 10.3g (0.06mol) of 1,2,2,6,6-pentamethylpiperidol Slowly added into the above three-necked flask in batches, heated to 70°C, and continued to stir for 2h. After the reaction is over, transfer the reaction solution to a 250 mL beaker, add 100 mL of absolute ethanol to the beaker, stir well, slowly add triethylamine dropwise to it in an ice bath, adjust the pH of the solution to neutrality, Add 100 mL of dry ether to the beaker, a white precipitate appears in the solution, filter and wash the filter cake twice with ether (the amount of ether for each time is 50 ml), take the filtrate and carry out atmospheric distillation (collect the fraction at 90 ° C) to obtain chlorine The intermediate product HC-1.
[0052] 2) Dissolve 0.6g (0.015mol) NaOH in 30mL deionized water, transfer it to a three-necked ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com