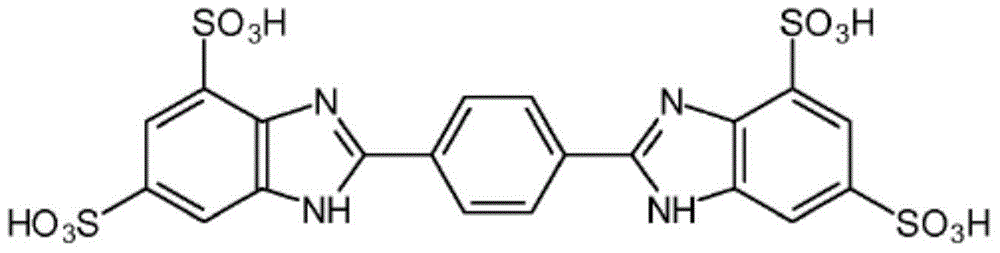
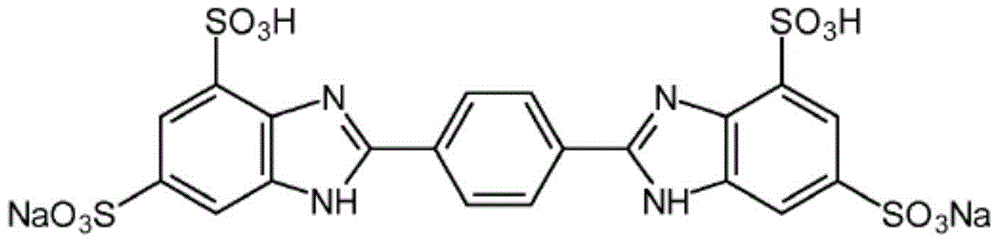
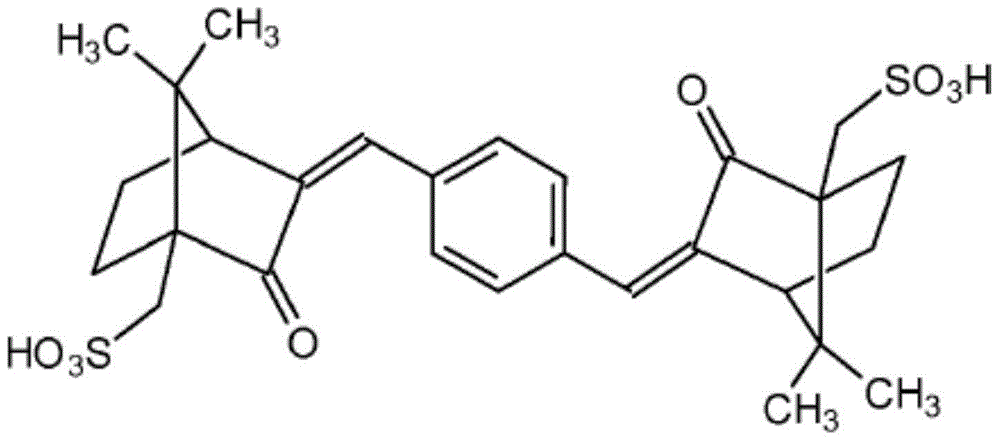
Compositions consisting of alkylamidothiazoles and uv-filter substances
A technology of alkylamide and ultraviolet filter, which is applied in the direction of drug combination, medical preparations containing active ingredients, organic active ingredients, etc. It can solve the problems of lack of active ingredients and treatment possibility, chronicity, distress, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0161] Method description for validity experiments
[0162] Thiazole effectiveness was determined by an enzymatic assay in which the conversion of levodopa to levodopaquinone by human tyrosinase was measured. In this method known from the literature (Winder, A.J., and Harris, H., New assays forthetyrosinehydroxylase and dopaoxidaseactivitiessoft tyrosinase. Eur.J. Analytical method). (1991), 198, 317-26), the reaction product levodopa quinone and MBTH (3-methyl-2-benzothiazolinone hydrazone) transforms into a pink substance, and the pink substance changes with time The increase was measured by absorption at 490 nm. In the table, the effectiveness data of several claimed substances are shown by way of example. This shows that the substances according to the invention are extremely effective pigmentation-inhibiting substances.
[0163]
[0164] Synthetic rules for exemplary selected alkylamidothiazoles:
[0165] 2-Bromo-2',4'-bismethoxycarbonyloxy-acetophenone:
[0166]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com