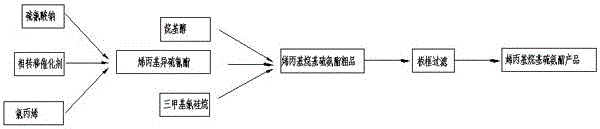
A kind of n-allyl-o-isobutyl thiocarbamate synthetic technique
A technology of allyl isothiocyanate and synthesis process, which is applied in the field of N-allyl-O-isobutyl thiourethane synthesis process, and can solve the problem that the content of allyl isobutyl thiourethane is not high , low conversion rate and other problems, to achieve the effect of strong reaction activity, improved environmental conditions, and reduced residual amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
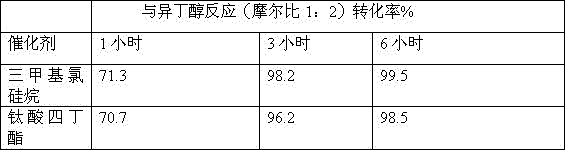
[0020] 0.25 moles of allyl isothiocyanate and 0.5 moles of isobutanol are heated together to 110°C under normal pressure, and catalyzed by 0.4 g / mole of trimethylchlorosilane or tetrabutyl titanate; proceed accordingly Reaction, conversion ratio is measured with gas chromatography; Reaction result is shown in Table 1:
[0021]
[0022] The data in Table 1 shows that using trimethylchlorosilane as a catalyst compared with tetrabutyl titanate or iron acetylacetonate as a catalyst, the conversion rate has been greatly improved after 3-6 hours of reaction.
Embodiment 2
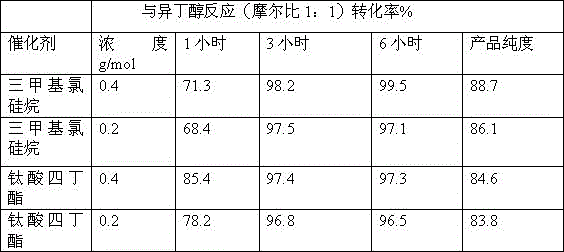
[0024] Carry out the reaction according to Experiment 1, and change the molar ratio of reaction with isobutanol to 1:1, and the test results are shown in Table 2:
[0025]
[0026] The data given in Table 2 shows that when the molar ratio of the reaction with isobutanol is 1:1, using trimethylchlorosilane as the catalyst, the conversion rate and purity of this reaction are relatively high in a short period of time.
Embodiment 3
[0028] The reaction of allyl isothiocyanate with propanol
[0029] Except that 0.25 mole of propanol is used instead of 0.25 mole of isobutanol, the experimental procedure is the same as experiment two; with 0.1g trimethylsilyl chloride (0.4g / mol allyl isothiocyanate); at 110 After heating at ℃ for 6 hours, the conversion rate of N-allyl-O-propyl-thiocarbamate was 97.2%, and the purity was 89.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com