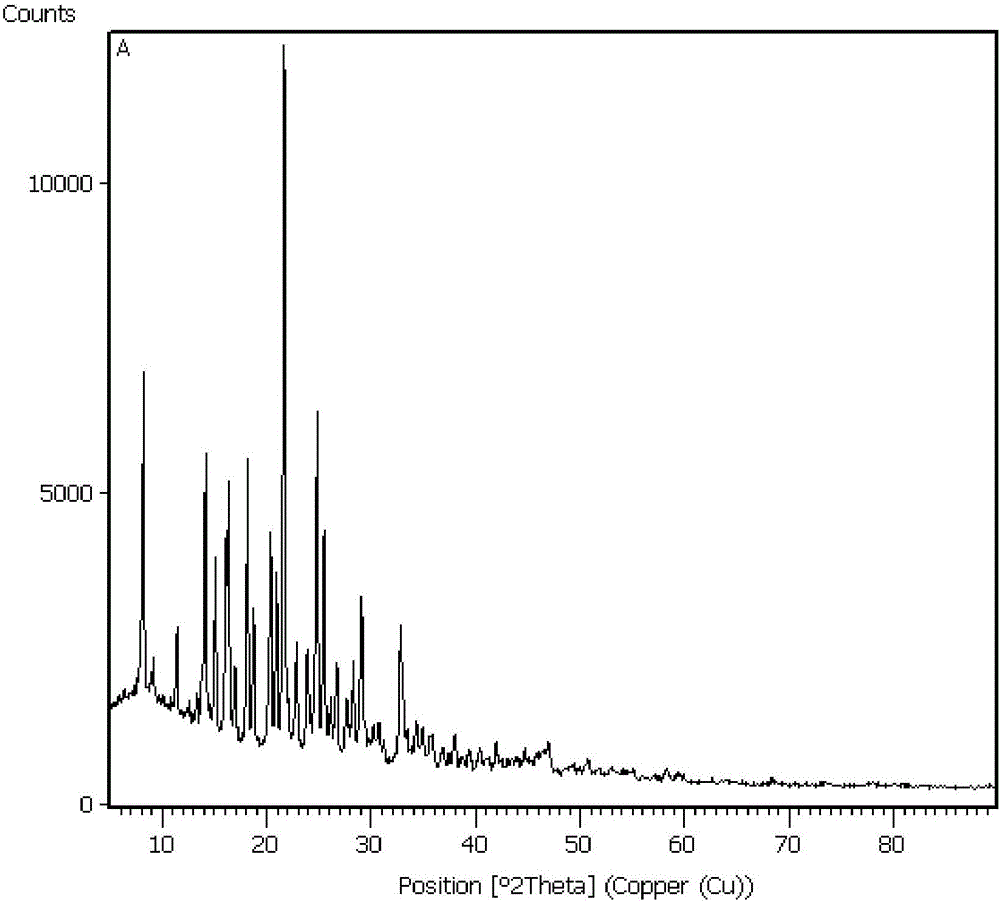
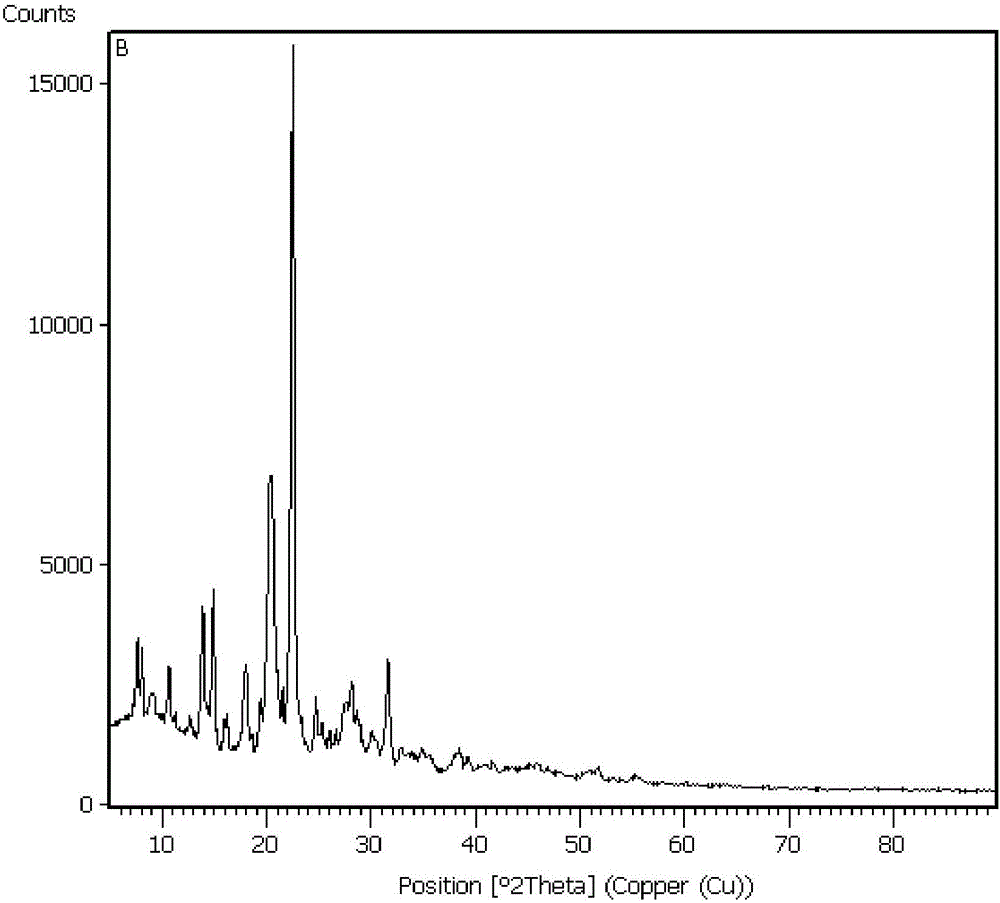
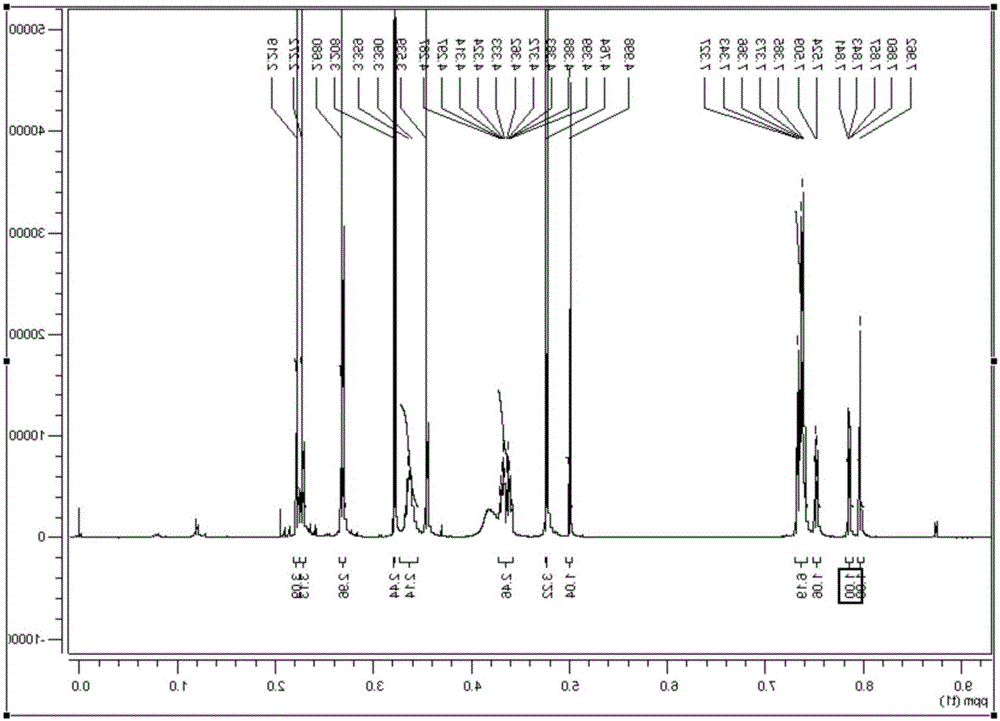
Preparation method for synthesizing nicardipine hydrochloride
A nicardipine hydrochloride and hydrochloric acid technology, applied in the field of medicine, can solve the problems of increasing product quality risks and post-processing difficulties, and achieve high yield and high purity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0030] Implementation Case 1: Synthesis of α-crystalline nicardipine hydrochloride with acetone as solvent
[0031] Pump 250kg of acetone into the PM-R-C-01 reaction tank, turn on the stirring, open the feeding port, and add 100kg of 3-amino-2-butenoic acid-2'-(N-benzyl-N-methyl)aminoethyl in sequence , 100kg 3-methyl-4-(3'-nitrophenyl)-3-butenoic acid methyl ester and cover the feeding port. Heat to 50°C, keep it warm for 8 hours, add 1kg of activated carbon for decolorization, filter, and cool the filtrate to 35°C, add 44kg of hydrochloric acid, stir and crystallize at 30-35°C for 12 hours, centrifuge and dry to obtain 148kg of α-crystalline nicardipine hydrochloride, Yellow-green crystalline powder, melting point: 182-184°C, HPLC content: 99.2%, yield 76.68%.
Embodiment example 2
[0032] Implementation Case 2: Synthesis of α-crystalline nicardipine hydrochloride with chloroform as solvent
[0033] Pump 250kg of chloroform into the PM-R-C-01 reaction tank, turn on the stirring, open the feeding port, and add 100kg of 3-amino-2-butenoic acid-2'-(N-benzyl-N-methyl)aminoethyl in sequence , 100kg 3-methyl-4-(3'-nitrophenyl)-3-butenoic acid methyl ester and cover the feeding port. Heat to 60°C, keep warm for 6 hours, add 1kg of activated carbon for decolorization, filter, and cool the filtrate to 35°C, add 44kg of hydrochloric acid, stir and crystallize at 30-35°C for 12 hours, centrifuge and dry to obtain 158.5kg of α-crystalline nicardipine hydrochloride , yellow-green crystalline powder, melting point: 181-183°C, HPLC content: 99.5%, yield 82.02%.
Embodiment example 3
[0034] Implementation Case 3: Synthesis of α-crystalline nicardipine hydrochloride with methanol as solvent
[0035] Pump 250kg of methanol into the PM-R-C-01 reaction tank, turn on the stirring, open the feeding port, and add 100kg of 3-amino-2-butenoic acid-2'-(N-benzyl-N-methyl)aminoethyl in sequence , 100kg 3-methyl-4-(3'-nitrophenyl)-3-butenoic acid methyl ester and cover the feeding port. Heat to 70°C, keep warm for 2 hours, add 1kg of activated carbon for decolorization, filter, concentrate the filtrate to viscous, add 250kg of chloroform to dissolve, cool down to 35°C, add 44kg of hydrochloric acid, stir and crystallize at 30-35°C for 12 hours, centrifuge and dry 135.6 kg of nicardipine hydrochloride in α-crystal form was obtained, yellow-green crystalline powder, melting point: 182-183° C., HPLC content: 99.8%, yield 70.02%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com