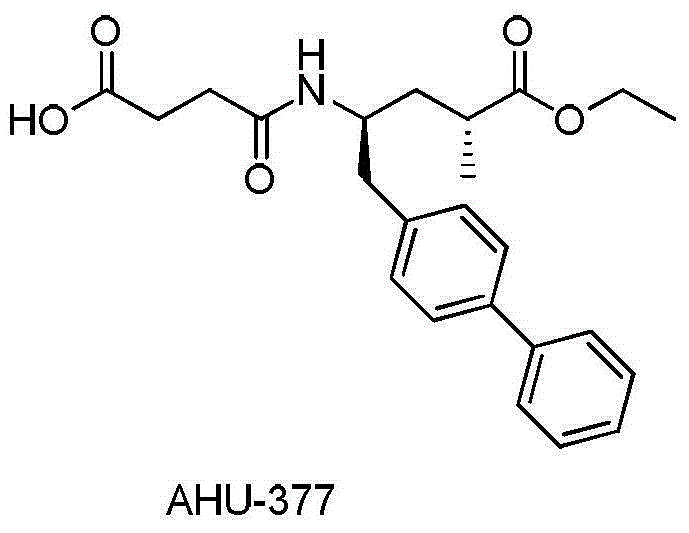
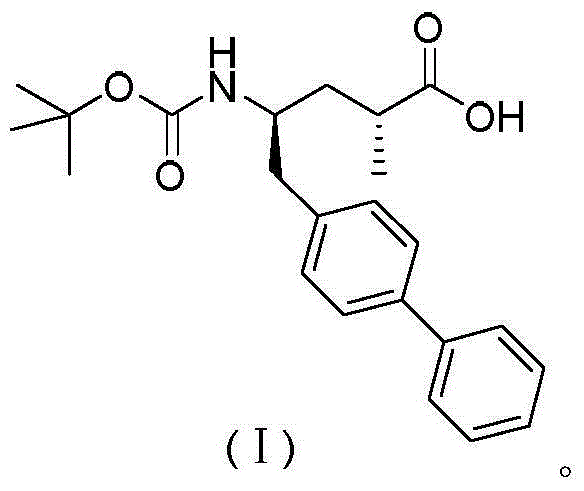
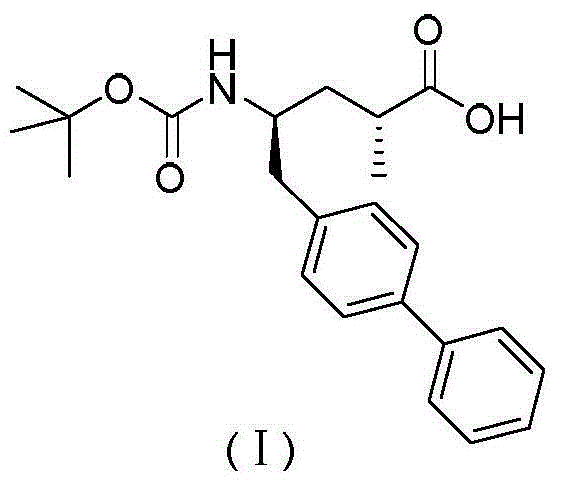
Preparation method and intermediate of AHU-377 intermediate and preparation method of intermediate
A technology of AHU-377 and intermediates, applied in the field of medicinal chemistry synthesis, can solve the problems of difficult removal of diastereomers, reduced yield and purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Synthesis of compound (Ⅲ-a)
[0067]
[0068] Dissolve (R)-4-phenyl-2-oxazolidinone (16.3g, 1 equivalent) in 180ml of dichloromethane, add triethylamine (15.2g, 1.5 equivalent) under stirring in an ice bath at 0°C, and 4- Dimethylaminopyridine (DMAP) (366 mg, 0.03 equivalents), then added propionyl chloride (9.2 g, 1 equivalents) dropwise, kept stirring at 0°C for 1 hour, diluted with dichloromethane, washed with water, washed with saturated sodium bicarbonate, organic The phase was dried over anhydrous sodium sulfate. The solvent was removed by evaporation under reduced pressure to obtain a crude product. The crude product was purified by column chromatography to obtain the target compound (Ⅲ-a) (20.1 g, yield 92%).
Embodiment 2
[0070] Synthesis of compound (Ⅲ-b)
[0071]
[0072] Dissolve (R)-4-benzyl-2-oxazolidinone (20 g, 1 equivalent) in 200 ml of dichloromethane, add triethylamine (17.1 g, 1.5 equivalents) while stirring in an ice bath at 0°C, and 4-di Aminopyridine (414 mg, 0.03 eq), then propionyl chloride (10.4 g, 1 eq) was added dropwise, kept stirring at 0°C for 1 hour, diluted with dichloromethane, washed with water, washed with saturated sodium bicarbonate, and the organic phase was washed with anhydrous Na2SO4 dried. The solvent was removed by evaporation under reduced pressure to obtain a crude product. The crude product was purified by column chromatography to obtain the target compound (Ⅲ-b) (24.3 g, yield 93%).
Embodiment 3
[0074] Synthesis of compound (Ⅲ-c)
[0075]
[0076] Dissolve (R)-4-isopropyl-2-oxazolidinone (14.3 g, 1 equivalent) in 150 ml of dichloromethane, and add triethylamine (15.2 g, 1.5 equivalents) under stirring in an ice bath at 0° C., 4 -Dimethylaminopyridine (366mg, 0.03 equivalents), then added propionyl chloride (9.2g, 1 equivalents) dropwise, kept stirring at 0°C for 1 hour, diluted with dichloromethane, washed with water, washed with saturated sodium bicarbonate, and the organic phase was washed with Dry over anhydrous sodium sulfate. The solvent was removed by evaporation under reduced pressure to obtain a crude product. The crude product was purified by column chromatography to obtain the target compound (Ⅲ-c) (18.3 g, yield 92%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com