CIK (cytokine-induced killer) cell culture fluid, CIK cell culture method and application of lentinan in CIK cell culture
A technology of cell culture and lentinan, applied in the biological field, can solve the problems of poor proliferation of CIK cells, achieve high cell surface antigen content, large amplification multiple, and promote proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
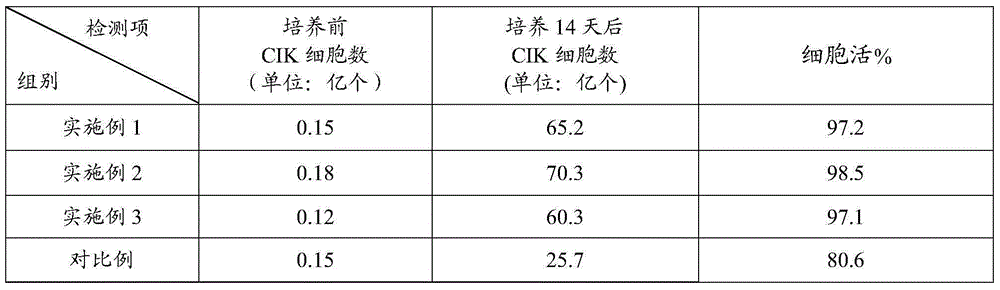
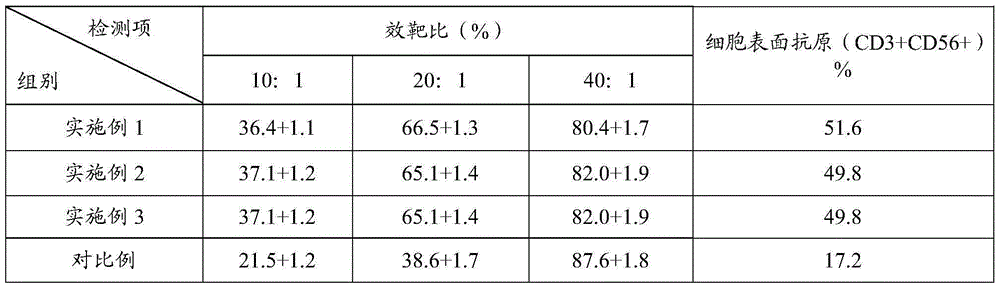
Examples
Embodiment 1
[0045] 1) Extract 40ml of peripheral blood, transfer the peripheral blood to a 50ml centrifuge tube, dilute and mix with 1 volume of normal saline, slowly add to Ficoll (Norway Axis-Shield company, product number: 1114547), centrifuge at 3500rpm for 30min ;
[0046] 2) After centrifugation, extract the PBMC band layer in the centrifuge tube, resuspend it with normal saline, and then centrifuge at 2000pm for 15min;
[0047] 3) After the centrifugation, remove the supernatant, resuspend the residue with normal saline again, and then centrifuge at 2000rpm for 15min;
[0048] 4) After centrifugation, remove the supernatant to obtain about 1.5×10 7 a PBMC;
[0049] 5) Use the culture medium to resuspend the PBMC obtained above to obtain a cell suspension; Company’s commercially available product), serum and RPMI-1640 medium, wherein, the content of IFN-γ is 1000U / ml, the content of CD3 stimulating monoclonal antibody is 80ng / ml, the content of IL-2 is 600U / ml, and the content of...
Embodiment 2
[0056] 1) Extract 40ml of peripheral blood, transfer the peripheral blood to a 50ml centrifuge tube, dilute and mix with 1 volume of normal saline, slowly add to Ficoll (Norway Axis-Shield company, product number: 1114547), centrifuge at 2800rpm for 20min ;
[0057] 2) After the centrifugation, extract the PBMC band layer in the centrifuge tube, resuspend with normal saline, and then centrifuge at 1800pm for 10min;
[0058] 3) After centrifugation, remove the supernatant, and resuspend the residue with normal saline again, then centrifuge at 1800rpm for 10min;
[0059] 4) After centrifugation, remove the supernatant to obtain 1.8×10 7 a PBMC;
[0060] 5) Use the culture medium to resuspend the PBMC obtained above to obtain a cell suspension; Company’s commercially available product), serum and RPMI-1640 medium, wherein, the content of IFN-γ is 500U / ml, the content of CD3 stimulating monoclonal antibody is 50ng / ml, the content of IL-2 is 500U / ml, and the content of lentinan ...
Embodiment 3
[0067]1) Extract 40ml of peripheral blood, transfer the peripheral blood to a 50ml centrifuge tube, dilute and mix with 1 volume of normal saline, slowly add to Ficoll (Norway Axis-Shield company, product number: 1114547), centrifuge at 2000rpm for 15min ;
[0068] 2) After centrifugation, extract the PBMC band layer in the centrifuge tube, resuspend it with normal saline, and then centrifuge at 1500pm for 5min;
[0069] 3) After the centrifugation, remove the supernatant, resuspend the residue with normal saline again, and then centrifuge at 1500rpm for 5min;
[0070] 4) After centrifugation, remove the supernatant to obtain 1.2×10 7 a PBMC;
[0071] 5) Use the culture medium to resuspend the PBMC obtained above to obtain a cell suspension; Company’s commercially available product), serum and RPMI-1640 medium, wherein, the content of IFN-γ is 200U / ml, the content of CD3 stimulating monoclonal antibody is 10ng / ml, the content of IL-2 is 100U / ml, and the content of lentinan ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com