Organic metal ruthenium compound crystal rich in boron and preparation method thereof
An organometallic and ruthenium compound technology, applied in the field of preparation of organometallic ruthenium compound crystals, can solve problems such as insufficient boron content, and achieve the effects of increasing boron content, mild reaction conditions and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
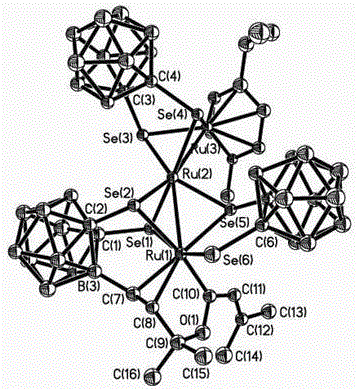
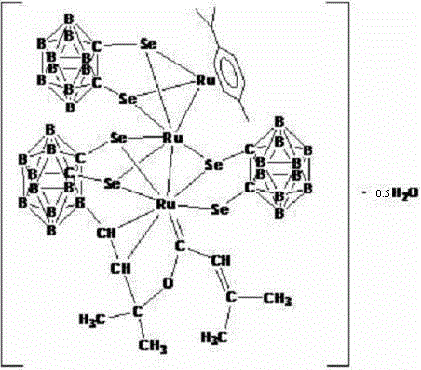
[0028] Under the protection of argon, 1,2-dicarbo-closed-dodecaborane (86mg, 0.6mmol) was dissolved in 20mL of anhydrous ether, and n-butyllithium (2.0mol×L -1 Cyclohexane solution) (0.6mL, 1.2mmol), selenium powder (158mg, 2.0mmol), after stirring and dissolving, add dichloro(p-methylisopropylphenyl) ruthenium (II) dimer ( 245mg, 0.4mmol) of tetrahydrofuran solution 40mL, ice-water bath temperature control 0 ° C, after 4 hours of reaction, the solvent was vacuum-dried; 20mL dichloromethane was used to dissolve the product, and HC≡CC(OH)(CH 3 ) 2(33.6mg, 0.4mmol) was added into the above-mentioned reaction flask, and after 22 hours of reaction at 25°C of temperature control, the reaction solution was concentrated to dryness; the target compound (83mg, 28%) was obtained by separation on a 200-300 mesh silica gel column (elution Agent: V (petroleum ether (60-90°C)) / V (dichloromethane)=2:1); the compound is dissolved in a mixed solvent of petroleum ether (60-90°C) and dichlorome...
Embodiment 2
[0030] Under the protection of argon, 1,2-dicarbo-closed-dodecaborane (86mg, 0.6mmol) was dissolved in 20mL of anhydrous ether, and n-butyllithium (2.0mol×L -1 Cyclohexane solution) (0.6mL, 1.2mmol), selenium powder (158mg, 2.0mmol), after stirring and dissolving, add dichloro(p-methylisopropylphenyl) ruthenium (II) dimer ( 245mg, 0.4mmol) of tetrahydrofuran solution 40mL, ice-water bath temperature control 0 ° C, after 4 hours of reaction, the solvent was vacuum-dried; 20mL dichloromethane was used to dissolve the product, and HC≡CC(OH)(CH 3 ) 2 (33.6mg, 0.4mmol) was added into the above-mentioned reaction flask, and after 20 hours of reaction at 30°C of temperature control, the reaction solution was concentrated to dryness; the target compound (77mg, 26%) was obtained by separation on a 200-300 mesh silica gel column (elution Agent: V (petroleum ether (60-90°C)) / V (dichloromethane)=2:1); the compound is dissolved in a mixed solvent of petroleum ether (60-90°C) and dichlorom...
Embodiment 3
[0032] Under the protection of argon, 1,2-dicarbo-closed-dodecaborane (86mg, 0.6mmol) was dissolved in 20mL of anhydrous ether, and n-butyllithium (2.0mol×L -1 Cyclohexane solution) (0.6mL, 1.2mmol), selenium powder (158mg, 2.0mmol), after stirring and dissolving, add dichloro(p-methylisopropylphenyl) ruthenium (II) dimer ( 245mg, 0.4mmol) of tetrahydrofuran solution 40mL, ice-water bath temperature control 0 ° C, after 4 hours of reaction, the solvent was vacuum-dried; 20mL dichloromethane was used to dissolve the product, and HC≡CC(OH)(CH 3 ) 2 (33.6mg, 0.4mmol) was added in the above-mentioned reaction bottle, and after 21 hours of reaction at 28°C of temperature control, the reaction solution was concentrated to dryness; the target compound (77mg, 26%) was obtained by separation on a 200-300 mesh silica gel column (elution Agent: V (petroleum ether (60-90°C)) / V (dichloromethane)=2:1); the compound is dissolved in a mixed solvent of petroleum ether (60-90°C) and dichlorome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com