Preparation method of rebamipide aqueous suspension
A technology of rebamipide and suspension, which is applied in the field of eye drop preparation, can solve problems such as irritation, sandy feeling in the eyes, and uncomfortable feeling of patients, and achieve high reproducibility, which is beneficial to long-term storage and process. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
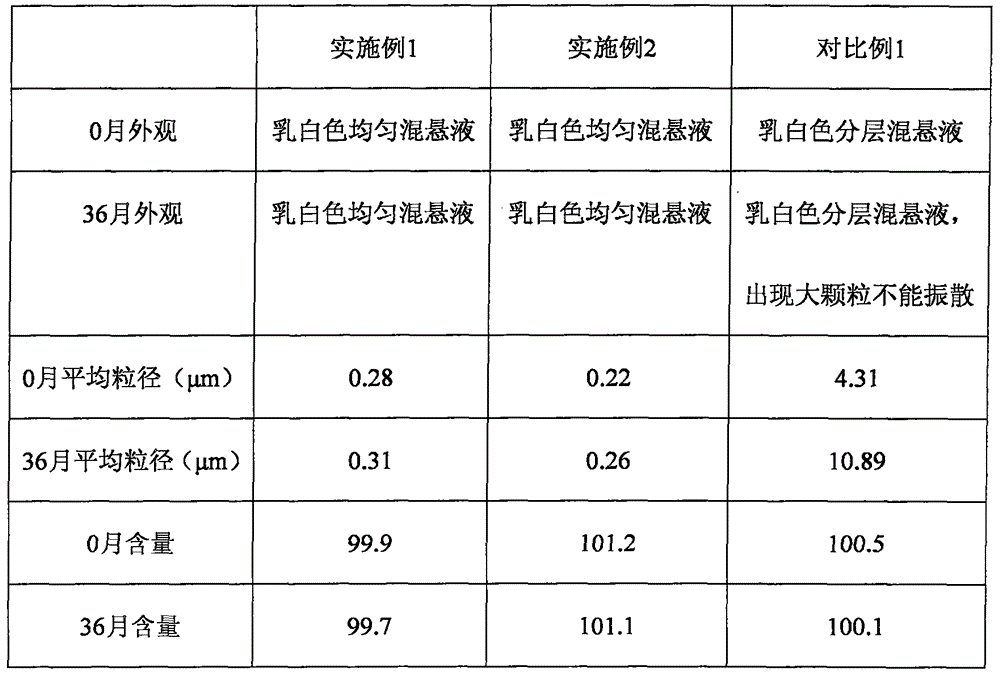
Embodiment 1
[0041] The preparation method of the rebamipide aqueous suspension provided in this embodiment comprises the following steps:
[0042] (1) According to the prescription requirements, weigh rebamipide and all excipients with an electronic balance.
[0043] (2) Dissolve poloxamer 188 and sodium citrate in part of the water for injection, turn on the shearing machine, adjust the speed to 10000-20000rmp, add rebamipide while cutting, after adding rebamipide Cut for another 5-10 minutes to obtain the initial solution of rebamipide concentrated aqueous suspension;
[0044] (3) Add the initial solution of rebamipide concentrated aqueous suspension into a high-pressure homogenizer, homogenize at a low pressure of 5-15MPa for 1-5 times, and homogenize at a high pressure of 50-60MPa for 8-18 times, and sterilize with damp heat at 110-130°C for 5 -30 minutes to obtain rebamipide concentrated aqueous suspension;
[0045] (4) Dissolving hydrochloric acid, sodium chloride and potas...
Embodiment 2
[0049] The preparation method of the rebamipide aqueous suspension provided in this embodiment comprises the following steps:
[0050] (1) According to the prescription requirements, weigh rebamipide and all excipients with an electronic balance.
[0051] (2) Dissolve poloxamer 188, polyethylene glycol 400 and sodium citrate in part of the water for injection, turn on the shearing machine, adjust the speed to 10000-20000rmp, and add rebamipide while cutting After adding bamipide, cut for 5-10 minutes to obtain the initial liquid of rebamipide concentrated aqueous suspension;
[0052] (3) Add the initial solution of rebamipide concentrated aqueous suspension into a high-pressure homogenizer, homogenize at a low pressure of 5-15MPa for 1-5 times, and homogenize at a high pressure of 50-60MPa for 8-18 times, and sterilize with damp heat at 110-130°C for 5 -30 minutes to obtain rebamipide concentrated aqueous suspension;
[0053] (4) Dissolving hydrochloric acid, sodium c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Osmotic pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com