Oral phenobarbital freeze-dried powder preparation and preparation method thereof
A technology of phenobarbital and freeze-dried powder, which is applied in the field of phenobarbital oral freeze-dried powder and its preparation, can solve problems such as insufficient dosage, difficulty, and influence on drug treatment compliance, and achieve good drug compliance, Rapid onset, considerable economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
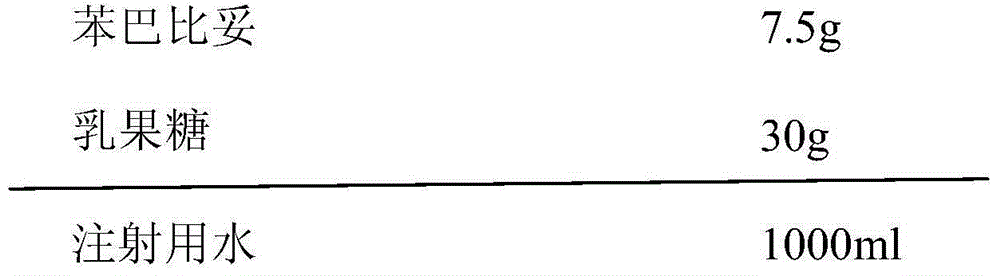
[0017] Each bottle contains phenobarbital 7.5mg, and the composition of 1000 bottles of phenobarbital orally disintegrating tablets is:
[0018]
[0019] (1) Add the prescribed amount of phenobarbital into the water for injection, heat to 60°C, then add the matrix agent to the above solution, stir to dissolve, adjust the pH of the solution to 6-7, and add sterilized water for injection to the full amount;
[0020] (2) Add gac in the prepared solution of step (1), the consumption of gac is 0.1g / mL of solution volume, filter;
[0021] (3) Place the filtrate obtained in step (2) in a freezer that has been cooled at -20°C, and freeze it for 3 hours, then lower it to -50°C, vacuumize, and heat up to -8 within 10 hours. ~-6°C, and maintain it for 6 hours, then raise the temperature to 22-30°C within 7-10 hours, and maintain it for 6 hours, to obtain phenobarbital oral freeze-dried powder.
Embodiment 2
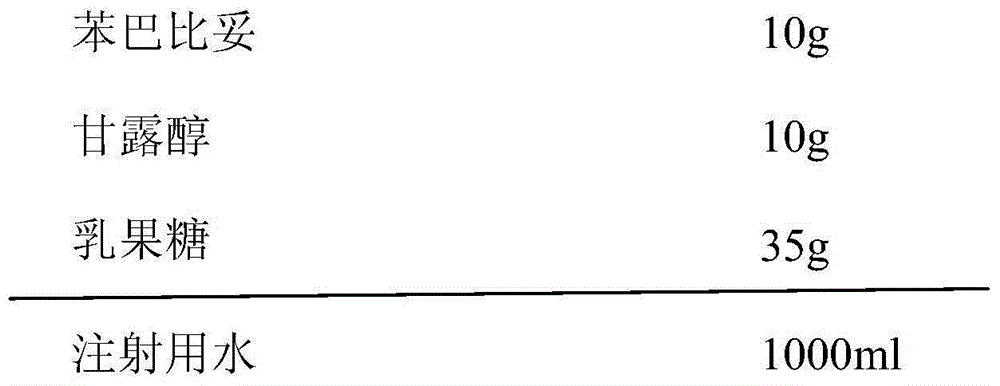
[0023] Each bottle contains phenobarbital 10mg, and the composition of 1000 bottles of phenobarbital orally disintegrating tablets is:
[0024]
[0025] (1) Add the prescribed amount of phenobarbital into the water for injection, heat to 60°C, then add the matrix agent to the above solution, stir to dissolve, adjust the pH of the solution, and add sterilized water for injection to the full amount;
[0026] (2) Add gac in the prepared solution of step (1), the consumption of gac is 0.1g / mL of solution volume, filter;
[0027] (3) Place the filtrate obtained in step (2) in a freezer that has been cooled at -20°C, and freeze it for 3 hours, then lower it to -50°C, vacuumize, and heat up to -8 within 10 hours. ~-6°C, and maintain it for 6 hours, then raise the temperature to 22-30°C within 7-10 hours, and maintain it for 6 hours, to obtain phenobarbital oral freeze-dried powder.
Embodiment 3
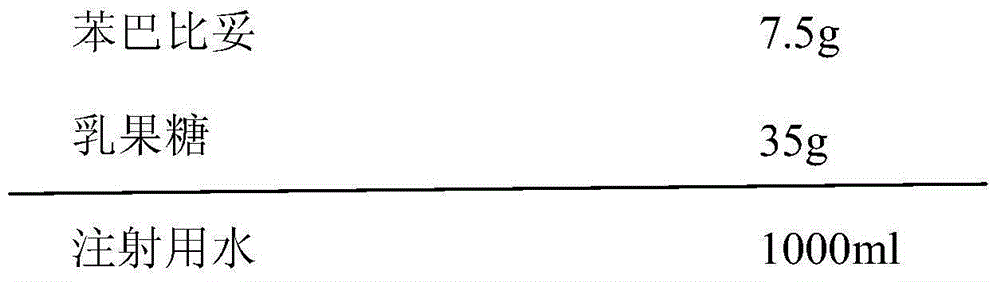
[0029] Each bottle contains phenobarbital 7.5mg, and the composition of 1000 bottles of phenobarbital orally disintegrating tablets is:
[0030]
[0031] (1) Add the prescribed amount of phenobarbital into the water for injection, heat to 60°C, then add the matrix agent to the above solution, stir to dissolve, adjust the pH of the solution to 6-7, and add sterilized water for injection to the full amount;
[0032] (2) Add gac in the prepared solution of step (1), the consumption of gac is 0.1g / mL of solution volume, filter;
[0033] (3) Place the filtrate obtained in step (2) in a freezer that has been cooled at -20°C, and freeze it for 3 hours, then lower it to -50°C, vacuumize, and heat up to -8 within 10 hours. ~-6°C, and maintain it for 6 hours, then raise the temperature to 22-30°C within 7-10 hours, and maintain it for 6 hours, to obtain phenobarbital oral freeze-dried powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com