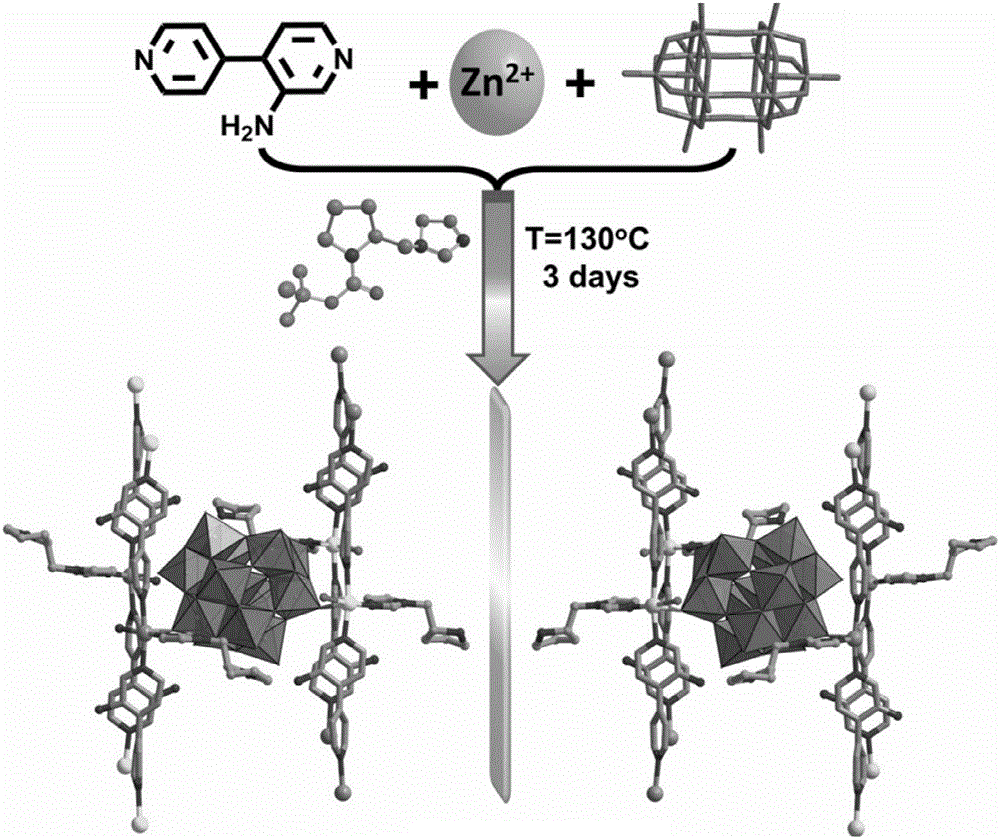
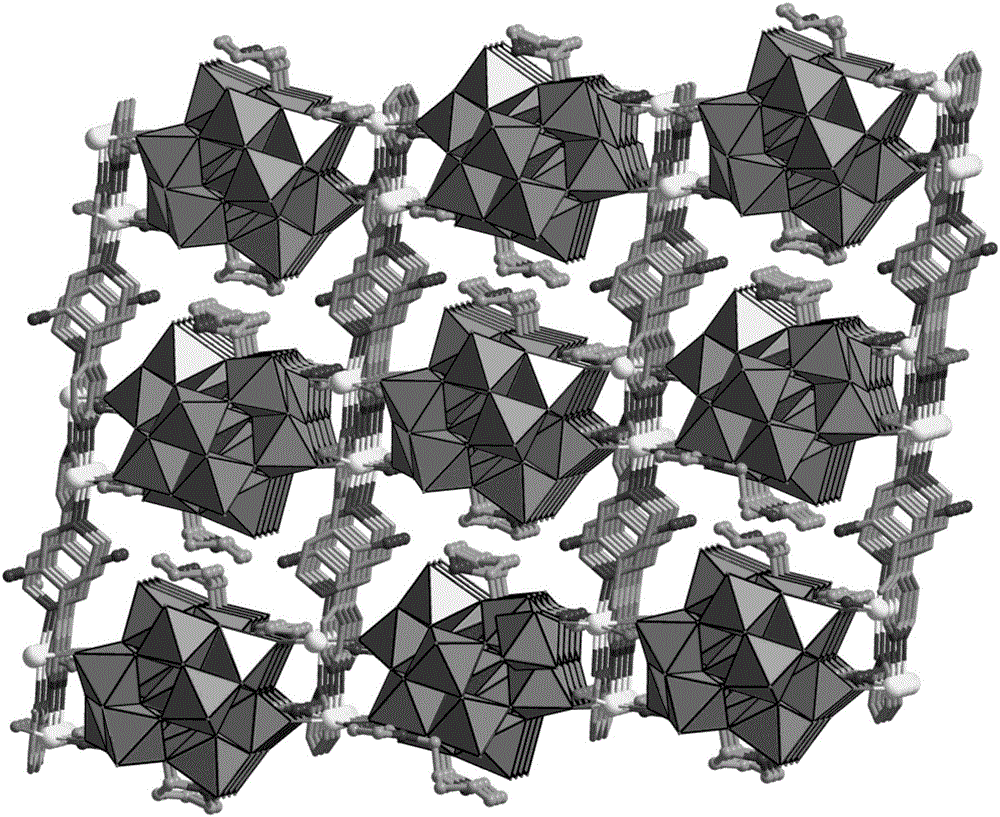
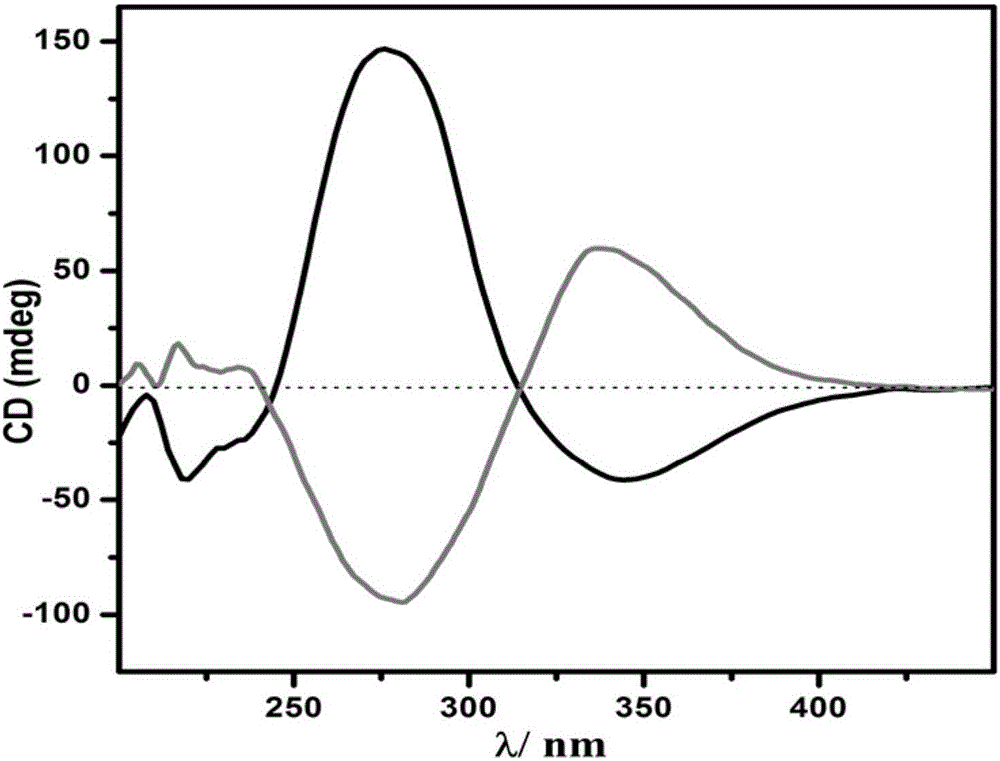
Preparation method of chiral POMOFs
A chiral, selected technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., to achieve simple synthesis, low price, good conversion rate and stereoselectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Na 4 W 10 o 32 ·8H 2 O (130mg, 0.05mmol), Zn (NO 3 ) 2 ·6H 2 O (29.8mg, 0.1mmol), 3-amino-4,4'-bipyridine (34.2mg, 0.2mmol) and L-N-tert-butoxycarbonyl-2-imidazole-1-pyrrolidine (25.0mg, 0.1mmol) Dissolve in a mixed solution of water (6.0mL) and acetonitrile (2.0mL) and use 1mol·L -1 HCl adjusted the pH value of the solution to 3.6, stirred evenly, placed in an oven, fired at 130°C for 72h, closed the oven, cooled to room temperature, colorless rod-shaped crystals were produced, filtered, and dried to obtain the target material POMOF (catalyst ZnW- PYI1), yield about 68%. Elemental analysis (%) for C 40 h 54 N 14 o 41 W 12 Zn 3 : C12.68, H1.44, N5.18, Zn5.18, W58.22; Found: C12.64, H1.41, N5.20, Zn5.22, W58.24.IR(KBr): 3440( s), 3123(w), 1619(s), 1532(s), 1247(s), 1103(w), 938(s), 872(s), 756(vs)cm-1 Table 1 shows the reaction performance test of the material in Example 1 asymmetrically catalyzing the one-pot synthesis of cyclocarbonate from olefins.
Embodiment 2
[0029] Na 4 W 10 o 32 ·8H 2 O (130mg, 0.05mmol), Zn (NO 3 ) 2 ·6H 2 O (29.8mg, 0.1mmol), 3-amino-4,4'-bipyridine (34.2mg, 0.2mmol) and D-N-tert-butoxycarbonyl-2-imidazole-1-pyrrolidine (25.0mg, 0.1mmol) Dissolve in a mixed solution of water (6.0mL) and acetonitrile (2.0mL) and use 1mol·L -1 HCl adjusted the pH value of the solution to 3.6, stirred evenly, placed in an oven, fired at 130°C for 72h, closed the oven, cooled to room temperature, colorless rod-shaped crystals were produced, filtered, and dried to obtain the target material POMOF (catalyst ZnW- PYI2), yield about 68%. Elemental analysis (%) for C 40 h 54 N 14 o 41 W 12 Zn 3 : C12.68, H1.44, N5.18, Zn5.18, W58.22; Found: C12.64, H1.42, N5.17, Zn5.20, W58.25 for ZnW-PYI2.IR(KBr): 3443(s), 3124(w), 1618(s), 1531(s), 1248(s), 1104(w), 939(s), 873(s), 757(vs)cm -1 Table 1 shows the reaction performance test of the material in Example 2 asymmetrically catalyzing the one-pot synthesis of cyclocarbonate from ...
Embodiment 3
[0031] Na 4 W 10 o 32 ·8H 2 O (130mg, 0.05mmol), NiCl 2 ·6H 2 O (23.8mg, 0.1mmol), 2,4,6-Tri-pyridin-4-yl-[1,3,5]triazine (31.2mg, 0.1mmol) and L-N-tert-butoxycarbonyl-2-imidazole- 1-Pyrrolidine (25.0mg, 0.1mmol) was dissolved in a mixed solution of water (4.0mL) and acetonitrile (2.0mL) and mixed with 1mol·L -1 HCl adjusted the pH value of the solution to 4.0, stirred evenly, placed in an oven, fired at 130°C for 72h, closed the oven, cooled to room temperature, light green massive crystals were produced, filtered, and dried to obtain the target material POMOF (catalyst NiW -PYI3), yield about 45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com