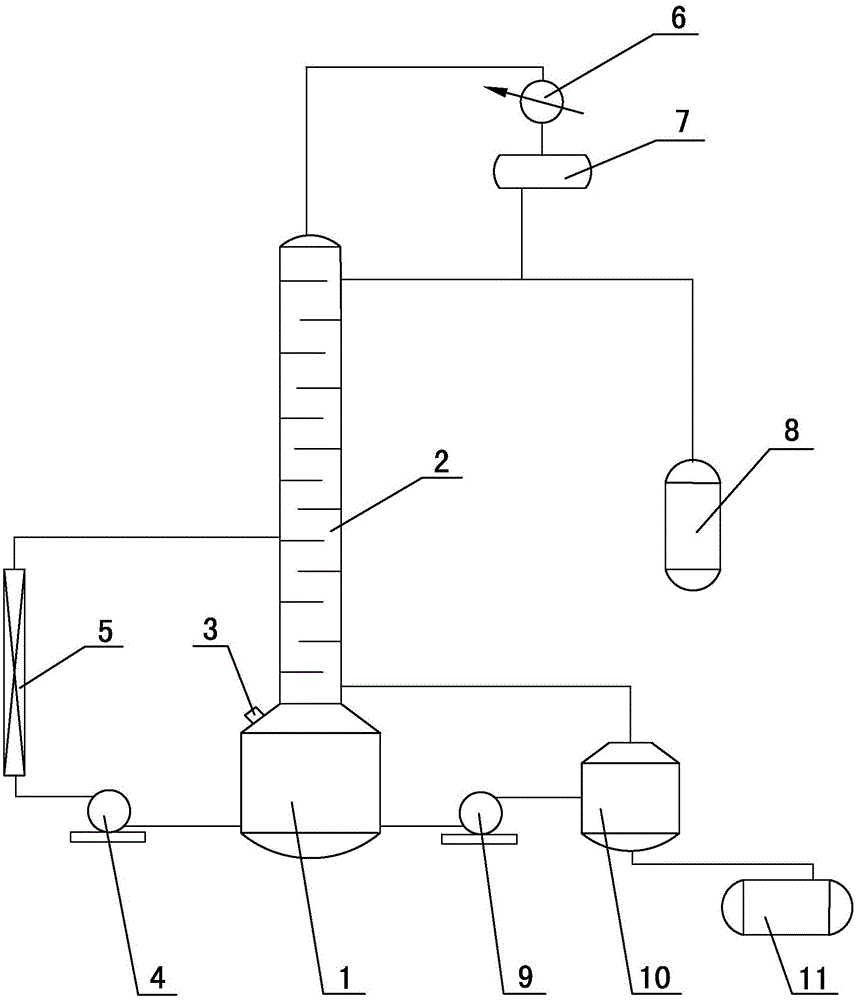
Method for continuously producing 2,3-dimethyl-1-butylene through 2,3-dimethyl-2-butylene
A technology of dimethyl and butene, which is used in organic chemistry, isomerization to produce hydrocarbons, distillation purification/separation, etc., can solve the problems of excessive generation and accumulation, product quality, reaction temperature and adverse effects of catalysts, and shorten the process. Process, low mechanical strength, and the effect of ensuring product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Throw 5kg of 2,3-dimethyl-2-butene into the distillation kettle, keep the temperature in the kettle at 60-80°C, pump the reaction tube with 60g of isomerization catalyst at a speed of 40-65L / h 2,3-dimethyl-2-butene is transported in the middle, the temperature of the reaction tube is maintained at 50-60°C, and then the reaction tube is returned to the kettle, and after 2.5 hours of reciprocating circulation, 2,3-dimethyl-1 -The content of butene reaches more than 5%; after 4 hours of circulation, the content ratio of 2,3-dimethyl-2-butene and 2,3-dimethyl-1-butene can reach a balance of 10:1-12 :1.
Embodiment 2
[0040] Change the temperature of the reaction tube in Example 1 to 60-70°C, and carry out the reaction under the same conditions as in Example 1. At this time, after 1.5 hours of circulation, the content of 2,3-dimethyl-1-butene The content ratio of 2,3-dimethyl-2-butene and 2,3-dimethyl-1-butene can reach a balance of 9:1-11:1 after circulation for 3 hours.
Embodiment 3
[0042] Change the temperature of the reaction tube in Example 1 to 40-50°C, and carry out the reaction under the same conditions as in Example 1. At this time, the content of 2,3-dimethyl-1-butene after 3.5 hours of circulation reaches More than 5%; after 6 hours of circulation, the content ratio of 2,3-dimethyl-2-butene to 2,3-dimethyl-1-butene can reach a balance of 10:1-12:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com